A pilot surveillance report of SARS-CoV-2 rapid antigen test results among volunteers in Germany, 1st week of July 2022
- PMID: 36279033
- PMCID: PMC9590393
- DOI: 10.1007/s15010-022-01931-7
A pilot surveillance report of SARS-CoV-2 rapid antigen test results among volunteers in Germany, 1st week of July 2022
Abstract
Purpose: We hypothesized that SARS-CoV-2 infection numbers reported by governmental institutions are underestimated due to high dark figures as only results from polymerase chain reaction (PCR) tests are incorporated in governmental statistics and testing capacities were further restricted as of July, 2022.
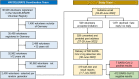
Methods: A point prevalence investigation was piloted by rapid antigen testing (RAT) among participants of the VACCELERATE volunteer registry. 2400 volunteers were contacted, of which 500 received a RAT including instructions for self-testing in the first week of July, 2022. Results were self-reported via e-mail.
Results: 419 valid RAT results were collected until July 7th, 2022. Between July-1 and July-7, 2022, 7/419 (1.67%) tests were positive. Compared to reports of the German Federal Government, our results suggest a more than twofold higher prevalence. Three out of seven positive individuals did not have a PCR test and are therefore likely not to be displayed in governmental statistics.
Conclusion: Our findings imply that the actual prevalence of SARS-CoV-2 may be higher than detected by current surveillance systems, so that current pandemic surveillance and testing strategies may be adapted.
Keywords: Pandemic preparedness; Point prevalence; Rapid antigen test; SARS-CoV-2; Self-testing; Surveillance.
© 2022. The Author(s).
Conflict of interest statement
JS has received research grants by the Ministry of Education and Research (BMBF) and Basilea Pharmaceuticals Inc.; has received speaker honoraria by Pfizer Inc. and Gilead; has been a consultant to Gilead, Produkt&Markt GmbH, Alvea Vax. and Micron Research, and has received travel grants by German Society for Infectious Diseases (DGI e.V.) and Meta-Alexander Foundation. JSG reports speaker honoraria for Gilead and Pfizer. BW, CT, CJ, JF have nothing to disclose. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Abbvie, Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pardes, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, Pulmocide, Shionogi; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley.
Figures

References
-
- Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19)-09.06.2022. Robert Koch-Institut: Berlin; 2022.
-
- Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19)-23.06.2022. Robert Koch-Institut: Berlin; 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

