The essential role of arterial pulse in venous return
- PMID: 36279504
- PMCID: PMC9722249
- DOI: 10.1152/ajpregu.00204.2022
The essential role of arterial pulse in venous return
Abstract
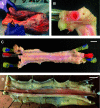
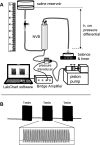
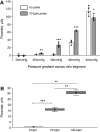
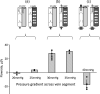
The close proximity of arteries and veins is a well-known anatomical finding documented in the extremities of all vertebrates. However, the physiological consequences of this arrangement are rarely given proper consideration nor are they covered in the textbook list of mechanisms that aid blood flow. We hypothesized that arterial pressure pulsations can significantly increase blood flow in the adjacent valve-containing vein segments. To demonstrate the existence of this mechanism, 10- to 15-cm sections of the bovine forelimb neurovascular bundle were isolated. The proximal and distal ends of the median artery and adjacent veins were cannulated, their tributaries were tied off, and the dissected bundle was then inserted into an airtight enclosure to mimic in vivo encasement by surrounding muscle. Pulsatile pressure was subsequently applied to the arterial segment while recording venous flow. At pressure settings mimicking physiological scenarios, arterial pulsations caused a highly significant increase in venous return. The amplitude of this effect was dependent on the arterial pulsation rate, stroke volume, and pressure gradient across the vein segment.
Keywords: neurovascular bundle; pulse pressure; venous return.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Supporting evidence for an "arterial pump" venous return mechanism in humans.J Appl Physiol (1985). 2023 Nov 1;135(5):1120-1125. doi: 10.1152/japplphysiol.00480.2023. Epub 2023 Oct 12. J Appl Physiol (1985). 2023. PMID: 37823204
-
Structural characteristics of the optic nerve head influencing human retinal venous pulsations.Exp Eye Res. 2016 Apr;145:341-346. doi: 10.1016/j.exer.2016.02.003. Epub 2016 Feb 15. Exp Eye Res. 2016. PMID: 26892807
-
Phase and amplitude of spontaneous retinal vein pulsations: An extended constant inflow and variable outflow model.Microvasc Res. 2016 Jul;106:67-79. doi: 10.1016/j.mvr.2016.03.005. Epub 2016 Mar 17. Microvasc Res. 2016. PMID: 26997658
-
Spontaneous retinal venous pulsation: aetiology and significance.J Neurol Neurosurg Psychiatry. 2003 Jan;74(1):7-9. doi: 10.1136/jnnp.74.1.7. J Neurol Neurosurg Psychiatry. 2003. PMID: 12486256 Free PMC article. Review.
-
Arterial pressure, vascular input impedance, and resistance as determinants of pulsatile blood flow in the umbilical artery.Eur J Obstet Gynecol Reprod Biol. 1999 Jun;84(2):119-25. doi: 10.1016/s0301-2115(98)00320-0. Eur J Obstet Gynecol Reprod Biol. 1999. PMID: 10428334 Review.
References
-
- Hall JE, Hall ME. Guyton and Hall: Textbook of Medical Physiology (14th ed). Philadelphia, PA: Elsevier; 2020.
-
- Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 146: 977–982, 1986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

