Safety and Immunogenicity of Intradermal Fractional Dose Administration of the mRNA-1273 Vaccine: A Proof-of-Concept Study
- PMID: 36279543
- PMCID: PMC9593280
- DOI: 10.7326/M22-2089
Safety and Immunogenicity of Intradermal Fractional Dose Administration of the mRNA-1273 Vaccine: A Proof-of-Concept Study
Conflict of interest statement
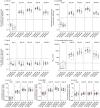
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
