Effects of SGLT2 inhibitor dapagliflozin in patients with type 2 diabetes on skeletal muscle cellular metabolism
- PMID: 36280113
- PMCID: PMC9636471
- DOI: 10.1016/j.molmet.2022.101620
Effects of SGLT2 inhibitor dapagliflozin in patients with type 2 diabetes on skeletal muscle cellular metabolism
Abstract
Objective: SGLT2 inhibitors increase urinary glucose excretion and have beneficial effects on cardiovascular and renal outcomes; the underlying mechanism may be metabolic adaptations due to urinary glucose loss. Here, we investigated the cellular and molecular effects of 5 weeks of dapagliflozin treatment on skeletal muscle metabolism in type 2 diabetes patients.
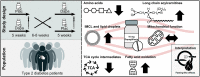
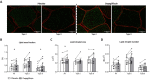
Methods: Twenty-six type 2 diabetes mellitus patients were randomized to a 5-week double-blind, cross-over study with 6-8-week wash-out. Skeletal muscle acetylcarnitine levels, intramyocellular lipid (IMCL) content and phosphocreatine (PCr) recovery rate were measured by magnetic resonance spectroscopy (MRS). Ex vivo mitochondrial respiration was measured in skeletal muscle fibers using high resolution respirometry. Intramyocellular lipid droplet and mitochondrial network dynamics were investigated using confocal microscopy. Skeletal muscle levels of acylcarnitines, amino acids and TCA cycle intermediates were measured. Expression of genes involved in fatty acid metabolism were investigated.
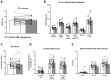
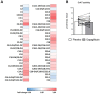
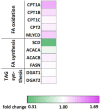
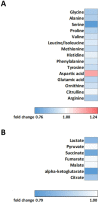
Results: Mitochondrial function, mitochondrial network integrity and citrate synthase and carnitine acetyltransferase activities in skeletal muscle were unaltered after dapagliflozin treatment. Dapagliflozin treatment increased intramyocellular lipid content (0.060 (0.011, 0.110) %, p = 0.019). Myocellular lipid droplets increased in size (0.03 μm2 (0.01-0.06), p < 0.05) and number (0.003 μm-2 (-0.001-0.007), p = 0.09) upon dapagliflozin treatment. CPT1A, CPT1B and malonyl CoA-decarboxylase mRNA expression was increased by dapagliflozin. Fasting acylcarnitine species and C4-OH carnitine levels (0.4704 (0.1246, 0.8162) pmoles∗mg tissue-1, p < 0.001) in skeletal muscle were higher after dapagliflozin treatment, while acetylcarnitine levels were lower (-40.0774 (-64.4766, -15.6782) pmoles∗mg tissue-1, p < 0.001). Fasting levels of several amino acids, succinate, alpha-ketoglutarate and lactate in skeletal muscle were significantly lower after dapagliflozin treatment.
Conclusion: Dapagliflozin treatment for 5 weeks leads to adaptive changes in skeletal muscle substrate metabolism favoring metabolism of fatty acid and ketone bodies and reduced glycolytic flux. The trial is registered with ClinicalTrials.gov, number NCT03338855.
Keywords: Acylcarnitines; Dapagliflozin; Mitochondrial function; Myocellular lipid metabolism; SGLT2i; TCA cycle Intermediates.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., Chertow G.M., Greene T., Hou F.F., et al. Dapagliflozin in patients with chronic kidney disease. The New England Journal of Medicine. 2020;383(15):1436–1446. - PubMed
-
- McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with heart failure and reduced Ejection fraction. The New England Journal of Medicine. 2019;381(21):1995–2008. - PubMed
-
- Bolinder J., Ljunggren O., Johansson L., Wilding J., Langkilde A.M., Sjostrom C.D., et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes, Obesity and Metabolism. 2014;16(2):159–169. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

