Profound Sensitivity of the Liver to the Direct Effect of Insulin Allows Peripheral Insulin Delivery to Normalize Hepatic but Not Muscle Glucose Uptake in the Healthy Dog
- PMID: 36280227
- PMCID: PMC9871195
- DOI: 10.2337/db22-0471
Profound Sensitivity of the Liver to the Direct Effect of Insulin Allows Peripheral Insulin Delivery to Normalize Hepatic but Not Muscle Glucose Uptake in the Healthy Dog
Abstract
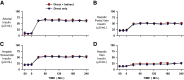
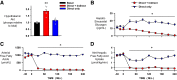
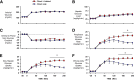
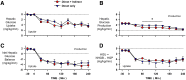
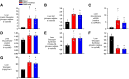
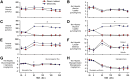
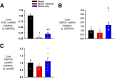
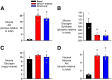
Endogenous insulin secretion is a key regulator of postprandial hepatic glucose metabolism, but this process is dysregulated in diabetes. Subcutaneous insulin delivery alters normal insulin distribution, causing relative hepatic insulin deficiency and peripheral hyperinsulinemia, a major risk factor for metabolic disease. Our aim was to determine whether insulin's direct effect on the liver is preeminent even when insulin is given into a peripheral vein. Postprandial-like conditions were created (hyperinsulinemia, hyperglycemia, and a positive portal vein to arterial glucose gradient) in healthy dogs. Peripheral (leg vein) insulin infusion elevated arterial and hepatic levels 8.0-fold and 2.8-fold, respectively. In one group, insulin's full effects were allowed. In another, insulin's indirect hepatic effects were blocked with the infusion of triglyceride, glucagon, and inhibitors of brain insulin action (intracerebroventricular) to prevent decreases in plasma free fatty acids and glucagon, while blocking increased hypothalamic insulin signaling. Despite peripheral insulin delivery the liver retained its full ability to store glucose, even when insulin's peripheral effects were blocked, whereas muscle glucose uptake markedly increased, creating an aberrant distribution of glucose disposal between liver and muscle. Thus, the healthy liver's striking sensitivity to direct insulin action can overcome the effect of relative hepatic insulin deficiency, whereas excess insulin in the periphery produces metabolic abnormalities in nonhepatic tissues.
© 2023 by the American Diabetes Association.
Figures

Similar articles
-
The Importance of the Mechanisms by Which Insulin Regulates Meal-Associated Liver Glucose Uptake in the Dog.Diabetes. 2021 Jun;70(6):1292-1302. doi: 10.2337/db20-1271. Epub 2021 Mar 23. Diabetes. 2021. PMID: 33757993 Free PMC article.
-
Insulin's direct effects on the liver dominate the control of hepatic glucose production.J Clin Invest. 2006 Feb;116(2):521-7. doi: 10.1172/JCI27073. J Clin Invest. 2006. PMID: 16453026 Free PMC article.
-
Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery.JCI Insight. 2019 Feb 26;5(7):e126974. doi: 10.1172/jci.insight.126974. JCI Insight. 2019. PMID: 30830873 Free PMC article.
-
New concepts in extracellular signaling for insulin action: the single gateway hypothesis.Recent Prog Horm Res. 1997;52:359-85; discussion 385-7. Recent Prog Horm Res. 1997. PMID: 9238859 Review.
-
Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein?Diabetologia. 2000 Jul;43(7):946-52. doi: 10.1007/s001250051474. Diabetologia. 2000. PMID: 10952470 Review.
Cited by
-
Insulin resistance in type 2 diabetes mellitus.Nat Rev Endocrinol. 2025 Jul;21(7):413-426. doi: 10.1038/s41574-025-01114-y. Epub 2025 Apr 17. Nat Rev Endocrinol. 2025. PMID: 40247011 Review.
-
Morning Engagement of Hepatic Insulin Receptors Improves Afternoon Hepatic Glucose Disposal and Storage.bioRxiv [Preprint]. 2024 Sep 27:2024.09.25.614969. doi: 10.1101/2024.09.25.614969. bioRxiv. 2024. Update in: Diabetes. 2025 Mar 01;74(3):270-281. doi: 10.2337/db24-0786. PMID: 39386695 Free PMC article. Updated. Preprint.
-
Improved Afternoon Hepatic Glucose Disposal and Storage Requires Morning Engagement of Hepatic Insulin Receptors.Diabetes. 2025 Mar 1;74(3):270-281. doi: 10.2337/db24-0786. Diabetes. 2025. PMID: 39602425
References
-
- Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia 1991;34:416–422 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

