Cerebrovascular insulin receptors are defective in Alzheimer's disease
- PMID: 36280236
- PMCID: PMC9897197
- DOI: 10.1093/brain/awac309
Cerebrovascular insulin receptors are defective in Alzheimer's disease
Abstract
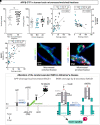
Central response to insulin is suspected to be defective in Alzheimer's disease. As most insulin is secreted in the bloodstream by the pancreas, its capacity to regulate brain functions must, at least partly, be mediated through the cerebral vasculature. However, how insulin interacts with the blood-brain barrier and whether alterations of this interaction could contribute to Alzheimer's disease pathophysiology both remain poorly defined. Here, we show that human and murine cerebral insulin receptors (INSRs), particularly the long isoform INSRα-B, are concentrated in microvessels rather than in the parenchyma. Vascular concentrations of INSRα-B were lower in the parietal cortex of subjects diagnosed with Alzheimer's disease, positively correlating with cognitive scores, leading to a shift towards a higher INSRα-A/B ratio, consistent with cerebrovascular insulin resistance in the Alzheimer's disease brain. Vascular INSRα was inversely correlated with amyloid-β plaques and β-site APP cleaving enzyme 1, but positively correlated with insulin-degrading enzyme, neprilysin and P-glycoprotein. Using brain cerebral intracarotid perfusion, we found that the transport rate of insulin across the blood-brain barrier remained very low (<0.03 µl/g·s) and was not inhibited by an insulin receptor antagonist. However, intracarotid perfusion of insulin induced the phosphorylation of INSRβ that was restricted to microvessels. Such an activation of vascular insulin receptor was blunted in 3xTg-AD mice, suggesting that Alzheimer's disease neuropathology induces insulin resistance at the level of the blood-brain barrier. Overall, the present data in post-mortem Alzheimer's disease brains and an animal model of Alzheimer's disease indicate that defects in the insulin receptor localized at the blood-brain barrier strongly contribute to brain insulin resistance in Alzheimer's disease, in association with β-amyloid pathology.
Keywords: Alzheimer’s disease; blood–brain barrier; insulin receptor; insulin resistance.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




Comment in
-
Cerebral insulin receptors: a nexus for vascular and metabolic contributions to Alzheimer's disease.Brain. 2023 Jan 5;146(1):8-9. doi: 10.1093/brain/awac433. Brain. 2023. PMID: 36402144 No abstract available.
Similar articles
-
Beta-amyloid pathology in human brain microvessel extracts from the parietal cortex: relation with cerebral amyloid angiopathy and Alzheimer's disease.Acta Neuropathol. 2019 May;137(5):801-823. doi: 10.1007/s00401-019-01967-4. Epub 2019 Feb 7. Acta Neuropathol. 2019. PMID: 30729296 Free PMC article.
-
Connective tissue growth factor (CTGF) expression in the brain is a downstream effector of insulin resistance- associated promotion of Alzheimer's disease beta-amyloid neuropathology.FASEB J. 2005 Dec;19(14):2081-2. doi: 10.1096/fj.05-4359fje. Epub 2005 Sep 26. FASEB J. 2005. PMID: 16186174
-
Altered cerebral vascular volumes and solute transport at the blood-brain barriers of two transgenic mouse models of Alzheimer's disease.Neuropharmacology. 2014 Jun;81:311-7. doi: 10.1016/j.neuropharm.2014.02.010. Epub 2014 Mar 12. Neuropharmacology. 2014. PMID: 24631967
-
Long-term use of metformin and Alzheimer's disease: beneficial or detrimental effects.Inflammopharmacology. 2023 Jun;31(3):1107-1115. doi: 10.1007/s10787-023-01163-7. Epub 2023 Feb 28. Inflammopharmacology. 2023. PMID: 36849855 Review.
-
Cerebrovascular transport of Alzheimer's amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier.Life Sci. 1996;59(18):1483-97. doi: 10.1016/0024-3205(96)00310-4. Life Sci. 1996. PMID: 8890929 Review.
Cited by
-
Evidence for an alternative insulin transporter at the blood-brain barrier.Aging Pathobiol Ther. 2022;4(4):100-108. doi: 10.31491/apt.2022.12.100. Epub 2022 Dec 29. Aging Pathobiol Ther. 2022. PMID: 36644126 Free PMC article.
-
Differential impact of eicosapentaenoic acid and docosahexaenoic acid in an animal model of Alzheimer's disease.J Lipid Res. 2024 Dec;65(12):100682. doi: 10.1016/j.jlr.2024.100682. Epub 2024 Oct 28. J Lipid Res. 2024. PMID: 39490923 Free PMC article.
-
Mediating role of Interleukin-6 in the predictive association of diabetes with Hippocampus atrophy, Amyloid, Tau, and Neurofilament pathology at pre-clinical stages of diabetes-related cognitive impairment.medRxiv [Preprint]. 2025 May 7:2025.05.06.25327092. doi: 10.1101/2025.05.06.25327092. medRxiv. 2025. Update in: Brain Behav Immun Health. 2025 Jun 16;47:101031. doi: 10.1016/j.bbih.2025.101031. PMID: 40385439 Free PMC article. Updated. Preprint.
-
The Role of Insulin Signaling in Hippocampal-Related Diseases: A Focus on Alzheimer's Disease.Int J Mol Sci. 2022 Nov 20;23(22):14417. doi: 10.3390/ijms232214417. Int J Mol Sci. 2022. PMID: 36430894 Free PMC article. Review.
-
APOE ɛ4 and Insulin Resistance Influence Path-Integration-Based Navigation through Distinct Large-Scale Network Mechanisms.Aging Dis. 2024 Nov 18;16(5):3154-3179. doi: 10.14336/AD.2024.0975. Aging Dis. 2024. PMID: 39656485 Free PMC article.
References
-
- Lewis GF, Brubaker PL. The discovery of insulin revisited: Lessons for the modern era. J Clin Invest. 2021;131(1):e1422399. - PMC - PubMed
-
- Polyzos SA, Mantzoros CS. Diabetes mellitus: 100 years since the discovery of insulin. Metabolism. 2021;118:154737. - PubMed
-
- Heni M, Kullmann S, Preissl H, Fritsche A, Häring H-U. Impaired insulin action in the human brain: Causes and metabolic consequences. Nat Rev Endocrinol. 2015;11:701–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

