American College of Rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision
- PMID: 36280238
- PMCID: PMC9811102
- DOI: 10.1136/ard-2022-223413
American College of Rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision
Abstract
Objective: In 2011, the American College of Rheumatology (ACR) and EULAR endorsed provisional criteria for remission in rheumatoid arthritis (RA), both Boolean-based and index-based. Based on recent studies indicating that a higher threshold for the patient global assessment (PtGA) may improve agreement between the two sets of criteria, our goals were to externally validate a revision of the Boolean remission criteria using a higher PtGA threshold and to validate the provisionally endorsed index-based criteria.
Methods: We used data from four randomised trials comparing biological disease-modifying antirheumatic drugs to methotrexate or placebo. We tested the higher proposed PtGA threshold of 2 cm (Boolean2.0) (range 0-10 cm) compared with the original threshold of 1 cm (Boolean1.0). We analysed agreement between the Boolean-based and index-based criteria (Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI)) for remission and examined how well each remission definition predicted later good physical function (Health Assessment Questionnaire (HAQ) score≤0.5) and radiographic non-progression.
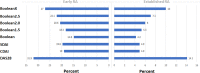
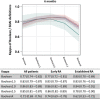
Results: Data from 2048 trial participants, 1101 with early RA and 947 with established RA, were included. The proportion of patients with disease in remission at 6 months after treatment initiation increased when using Boolean2.0 compared with Boolean1.0, from 14.8% to 20.6% in early RA and 4.2% to 6.0% in established RA. Agreement between Boolean2.0 and the SDAI or CDAI remission criteria was better than for Boolean1.0, particularly in early disease. Boolean2.0, SDAI, and CDAI remission criteria had similar positive likelihood ratios (LRs) to predict radiographic nonprogression and a HAQ score of ≤0.5 (positive LR 3.8-4.3). The omission of PtGA (BooleanX) worsened the prediction of good functional outcomes.
Conclusion: Using the Boolean 2.0 criteria classifies, more patients as achieving remission and increases the agreement with index-based remission criteria without jeopardising predictive value for radiographic or functional outcomes. This revised Boolean definition and the previously provisionally endorsed index-based criteria were endorsed by ACR and EULAR.
Keywords: Arthritis, Rheumatoid; Methotrexate.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
American College of Rheumatology/EULAR Remission Criteria for Rheumatoid Arthritis: 2022 Revision.Arthritis Rheumatol. 2023 Jan;75(1):15-22. doi: 10.1002/art.42347. Epub 2022 Oct 23. Arthritis Rheumatol. 2023. PMID: 36274193 Free PMC article.
-
Testing different thresholds for patient global assessment in defining remission for rheumatoid arthritis: are the current ACR/EULAR Boolean criteria optimal?Ann Rheum Dis. 2020 Apr;79(4):445-452. doi: 10.1136/annrheumdis-2019-216529. Epub 2020 Feb 5. Ann Rheum Dis. 2020. PMID: 32024651 Free PMC article.
-
Comparative Assessment of the Different American College of Rheumatology/European League Against Rheumatism Remission Definitions for Rheumatoid Arthritis for Their Use as Clinical Trial End Points.Arthritis Rheumatol. 2017 Mar;69(3):518-528. doi: 10.1002/art.39945. Arthritis Rheumatol. 2017. PMID: 27696724
-
American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials.Ann Rheum Dis. 2011 Mar;70(3):404-13. doi: 10.1136/ard.2011.149765. Ann Rheum Dis. 2011. PMID: 21292833
-
American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials.Arthritis Rheum. 2011 Mar;63(3):573-86. doi: 10.1002/art.30129. Arthritis Rheum. 2011. PMID: 21294106 Free PMC article.
Cited by
-
Efficacy and safety of filgotinib in patients with rheumatoid arthritis: week 156 interim results from a long-term extension study.RMD Open. 2024 Oct 24;10(4):e004476. doi: 10.1136/rmdopen-2024-004476. RMD Open. 2024. PMID: 39455065 Free PMC article. Clinical Trial.
-
Predictors of Boolean2.0 remission in rheumatoid arthritis identified using smart disease management system data.Sci Rep. 2025 Apr 21;15(1):13676. doi: 10.1038/s41598-025-97084-2. Sci Rep. 2025. PMID: 40258859 Free PMC article.
-
Real-World Effectiveness of Upadacitinib for Treatment of Rheumatoid Arthritis in Canadian Patients: Interim Results from the Prospective Observational CLOSE-UP Study.Rheumatol Ther. 2024 Jun;11(3):563-582. doi: 10.1007/s40744-024-00651-8. Epub 2024 Mar 11. Rheumatol Ther. 2024. PMID: 38467912 Free PMC article.
-
Average annual costs of Rheumatoid Arthritis estimated by inverse probability weighting and their influence factors: A cross-sectional study based on Chinese Registry of Rheumatoid arthritis (CREDIT) Cohort.PLoS One. 2025 Aug 25;20(8):e0330261. doi: 10.1371/journal.pone.0330261. eCollection 2025. PLoS One. 2025. PMID: 40853930 Free PMC article.
-
The Uncoupling of Disease Activity from Joint Structural Progression in Patients with Rheumatoid Arthritis Treated with Filgotinib.Rheumatol Ther. 2025 Feb;12(1):53-66. doi: 10.1007/s40744-024-00725-7. Epub 2024 Nov 26. Rheumatol Ther. 2025. PMID: 39592547 Free PMC article.
References
-
- Scott D, van Riel P, van der Heijde D. Assessing disease activity in rheumatoid arthritis: the EULAR Handbook of standard methods. In: On behalf of the EULAR standing Committee for international clinical studies including therapeutic trials. Zürich: EULAR, 1993.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

