Emerging links between endoplasmic reticulum stress responses and acute kidney injury
- PMID: 36280391
- PMCID: PMC9722262
- DOI: 10.1152/ajpcell.00370.2022
Emerging links between endoplasmic reticulum stress responses and acute kidney injury
Abstract
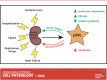
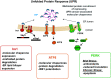
All cell types must maintain homeostasis under periods of stress. To prevent the catastrophic effects of stress, all cell types also respond to stress by inducing protective pathways. Within the cell, the endoplasmic reticulum (ER) is exquisitely stress-sensitive, primarily because this organelle folds, posttranslationally processes, and sorts one-third of the proteome. In the 1990s, a specialized ER stress response pathway was discovered, the unfolded protein response (UPR), which specifically protects the ER from damaged proteins and toxic chemicals. Not surprisingly, UPR-dependent responses are essential to maintain the function and viability of cells continuously exposed to stress, such as those in the kidney, which have high metabolic demands, produce myriad protein assemblies, continuously filter toxins, and synthesize ammonia. In this mini-review, we highlight recent articles that link ER stress and the UPR with acute kidney injury (AKI), a disease that arises in ∼10% of all hospitalized individuals and nearly half of all people admitted to intensive care units. We conclude with a discussion of prospects for treating AKI with emerging drugs that improve ER function.
Keywords: chemical chaperone; molecular chaperone; proteostasis; renal physiology; unfolded protein response (UPR).
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
Similar articles
-
Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury.Ann Med. 2018 Aug;50(5):381-390. doi: 10.1080/07853890.2018.1489142. Epub 2018 Jul 11. Ann Med. 2018. PMID: 29895209 Free PMC article. Review.
-
The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors.J Cell Mol Med. 2020 Nov;24(22):12910-12919. doi: 10.1111/jcmm.15999. Epub 2020 Oct 16. J Cell Mol Med. 2020. PMID: 33067928 Free PMC article. Review.
-
Pathophysiological Role of Organelle Stress/Crosstalk in AKI-to-CKD Transition.Semin Nephrol. 2019 Nov;39(6):581-588. doi: 10.1016/j.semnephrol.2019.10.007. Semin Nephrol. 2019. PMID: 31836040 Review.
-
Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease.Nat Rev Nephrol. 2014 Jul;10(7):369-78. doi: 10.1038/nrneph.2014.67. Epub 2014 Apr 22. Nat Rev Nephrol. 2014. PMID: 24752014 Review.
-
Organelle Stress and Crosstalk in Kidney Disease.Kidney360. 2020 Aug 7;1(10):1157-1164. doi: 10.34067/KID.0002442020. eCollection 2020 Oct 29. Kidney360. 2020. PMID: 35368784 Free PMC article. Review.
Cited by
-
Renoprotective Effects of Phloretin and TUDCA via Simultaneous Inhibition of TLR4/MyD88/NF-κB and BiP/PERK/CHOP Pathways in AKI Under Diabetic Condition.Appl Biochem Biotechnol. 2025 Jul 3. doi: 10.1007/s12010-025-05315-z. Online ahead of print. Appl Biochem Biotechnol. 2025. PMID: 40608259
-
Inhibition of MiR-106b-5p mediated by exosomes mitigates acute kidney injury by modulating transmissible endoplasmic reticulum stress and M1 macrophage polarization.J Cell Mol Med. 2023 Oct;27(19):2876-2889. doi: 10.1111/jcmm.17848. Epub 2023 Jul 20. J Cell Mol Med. 2023. PMID: 37471571 Free PMC article.
-
Diallyl Trisulfide Attenuates Ischemia-Reperfusion-Induced ER Stress and Kidney Dysfunction in Aged Female Mice.Cells. 2025 Mar 12;14(6):420. doi: 10.3390/cells14060420. Cells. 2025. PMID: 40136669 Free PMC article.
-
Puerarin alleviates renal ischemia/reperfusion injury by inhibiting apoptosis and endoplasmic reticulum stress via Nrf2/HO-1 pathway.Iran J Basic Med Sci. 2025;28(2):187-193. doi: 10.22038/ijbms.2024.80438.17412. Iran J Basic Med Sci. 2025. PMID: 39850122 Free PMC article.
-
Research progress and advances in endoplasmic reticulum stress regulation of acute kidney injury.Ren Fail. 2024 Dec;46(2):2433160. doi: 10.1080/0886022X.2024.2433160. Epub 2024 Nov 25. Ren Fail. 2024. PMID: 39586579 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

