Function of bidirectional sensitivity in the otolith organs established by transcription factor Emx2
- PMID: 36280667
- PMCID: PMC9592604
- DOI: 10.1038/s41467-022-33819-3
Function of bidirectional sensitivity in the otolith organs established by transcription factor Emx2
Abstract
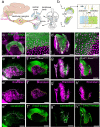
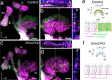
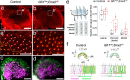
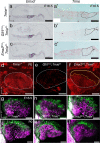
Otolith organs of the inner ear are innervated by two parallel afferent projections to the brainstem and cerebellum. These innervations were proposed to segregate across the line of polarity reversal (LPR) within each otolith organ, which divides the organ into two regions of hair cells (HC) with opposite stereociliary orientation. The relationship and functional significance of these anatomical features are not known. Here, we show regional expression of Emx2 in otolith organs, which establishes LPR, mediates the neuronal segregation across LPR and constitutes the bidirectional sensitivity function. Conditional knockout (cKO) of Emx2 in HCs lacks LPR. Tmie cKO, in which mechanotransduction was abolished selectively in HCs within the Emx2 expression domain also lacks bidirectional sensitivity. Analyses of both mutants indicate that LPR is specifically required for mice to swim comfortably and to traverse a balance beam efficiently, but LPR is not required for mice to stay on a rotating rod.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Emx2 lineage tracing reveals antecedent patterns of planar polarity in the mouse inner ear.Development. 2024 May 15;151(10):dev202425. doi: 10.1242/dev.202425. Epub 2024 May 16. Development. 2024. PMID: 38682291 Free PMC article.
-
Contributions of mirror-image hair cell orientation to mouse otolith organ and zebrafish neuromast function.Elife. 2024 Nov 12;13:RP97674. doi: 10.7554/eLife.97674. Elife. 2024. PMID: 39531034 Free PMC article.
-
Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells.Elife. 2017 Mar 7;6:e23661. doi: 10.7554/eLife.23661. Elife. 2017. PMID: 28266911 Free PMC article.
-
A balance of form and function: planar polarity and development of the vestibular maculae.Semin Cell Dev Biol. 2013 May;24(5):490-8. doi: 10.1016/j.semcdb.2013.03.001. Epub 2013 Mar 15. Semin Cell Dev Biol. 2013. PMID: 23507521 Free PMC article. Review.
-
The Superiority of the Otolith System.Audiol Neurootol. 2020;25(1-2):35-41. doi: 10.1159/000504595. Epub 2020 Jan 10. Audiol Neurootol. 2020. PMID: 31927546 Free PMC article. Review.
Cited by
-
Fish hearing revealed: Do we understand hearing in critical fishes and marine tetrapods.J Acoust Soc Am. 2023 Nov 1;154(5):3019-3026. doi: 10.1121/10.0022355. J Acoust Soc Am. 2023. PMID: 37955566 Free PMC article.
-
Anatomical and molecular insights into avian inner ear sensory hair cell regeneration.Dev Biol. 2025 Sep;525:13-25. doi: 10.1016/j.ydbio.2025.05.021. Epub 2025 May 23. Dev Biol. 2025. PMID: 40414451 Review.
-
Cerebellum in Alzheimer's disease and other neurodegenerative diseases: an emerging research frontier.MedComm (2020). 2024 Jul 13;5(7):e638. doi: 10.1002/mco2.638. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 39006764 Free PMC article. Review.
-
Emx2 lineage tracing reveals antecedent patterns of planar polarity in the mouse inner ear.Development. 2024 May 15;151(10):dev202425. doi: 10.1242/dev.202425. Epub 2024 May 16. Development. 2024. PMID: 38682291 Free PMC article.
-
Notch1 is Required to Maintain Supporting Cell Identity and Vestibular Function during Maturation of the Mammalian Balance Organs.J Neurosci. 2025 Feb 26;45(9):e1365242024. doi: 10.1523/JNEUROSCI.1365-24.2024. J Neurosci. 2025. PMID: 39779370 Free PMC article.
References
-
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J. Neurophysiol. 2005;93:251–266. - PubMed
-
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J. Neurophysiol. 2005;93:267–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

