Geometric engineering of organoid culture for enhanced organogenesis in a dish
- PMID: 36280722
- PMCID: PMC10027401
- DOI: 10.1038/s41592-022-01643-8
Geometric engineering of organoid culture for enhanced organogenesis in a dish
Abstract
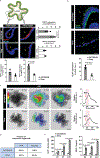
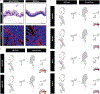
Here, we introduce a facile, scalable engineering approach to enable long-term development and maturation of organoids. We have redesigned the configuration of conventional organoid culture to develop a platform that converts single injections of stem cell suspensions to radial arrays of organoids that can be maintained for extended periods without the need for passaging. Using this system, we demonstrate accelerated production of intestinal organoids with significantly enhanced structural and functional maturity, and their continuous development for over 4 weeks. Furthermore, we present a patient-derived organoid model of inflammatory bowel disease (IBD) and its interrogation using single-cell RNA sequencing to demonstrate its ability to reproduce key pathological features of IBD. Finally, we describe the extension of our approach to engineer vascularized, perfusable human enteroids, which can be used to model innate immune responses in IBD. This work provides an immediately deployable platform technology toward engineering more realistic organ-like structures in a dish.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures












Comment in
-
Redesigning hydrogel geometry for enhanced organoids.Nat Methods. 2022 Nov;19(11):1347-1348. doi: 10.1038/s41592-022-01656-3. Nat Methods. 2022. PMID: 36280723 No abstract available.
References
-
- Clevers H Modeling development and disease with organoids. Cell 165, 1586–1597 (2016). - PubMed
-
- Lancaster MA & Knoblich JA Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 (2014). - PubMed
-
- Dutta D, Heo I & Clevers H Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med 23, 393–410 (2017). - PubMed
-
- Broutier L et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc 11, 1724–1743 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

