The role of insufficient sleep and circadian misalignment in obesity
- PMID: 36280789
- PMCID: PMC9590398
- DOI: 10.1038/s41574-022-00747-7
The role of insufficient sleep and circadian misalignment in obesity
Abstract
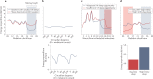
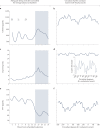
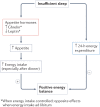
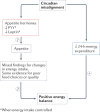
Traditional risk factors for obesity and the metabolic syndrome, such as excess energy intake and lack of physical activity, cannot fully explain the high prevalence of these conditions. Insufficient sleep and circadian misalignment predispose individuals to poor metabolic health and promote weight gain and have received increased research attention in the past 10 years. Insufficient sleep is defined as sleeping less than recommended for health benefits, whereas circadian misalignment is defined as wakefulness and food intake occurring when the internal circadian system is promoting sleep. This Review discusses the impact of insufficient sleep and circadian misalignment in humans on appetite hormones (focusing on ghrelin, leptin and peptide-YY), energy expenditure, food intake and choice, and risk of obesity. Some potential strategies to reduce the adverse effects of sleep disruption on metabolic health are provided and future research priorities are highlighted. Millions of individuals worldwide do not obtain sufficient sleep for healthy metabolic functions. Furthermore, modern working patterns, lifestyles and technologies are often not conducive to adequate sleep at times when the internal physiological clock is promoting it (for example, late-night screen time, shift work and nocturnal social activities). Efforts are needed to highlight the importance of optimal sleep and circadian health in the maintenance of metabolic health and body weight regulation.
© 2022. Springer Nature Limited.
Conflict of interest statement
K.P.W. reports research support and donated materials from DuPont Nutrition & Biosciences, Grain Processing Corporation and Friesland Campina Innovation Centre. K.P.W. also reports financial relationships, including consulting with or without receiving fees and/or serving on the advisory boards for Circadian Therapeutics, LTD., Circadian Biotherapies, Inc., Philips Respironics, and the U.S. Army Medical Research and Materiel Command–Walter Reed Army Institute of Research. The other authors declare no competing interests.
Figures

References
-
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

