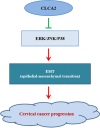
CLCA2 overexpression suppresses epithelial-to-mesenchymal transition in cervical cancer cells through inactivation of ERK/JNK/p38-MAPK signaling pathways
- PMID: 36280802
- PMCID: PMC9594891
- DOI: 10.1186/s12860-022-00440-7
CLCA2 overexpression suppresses epithelial-to-mesenchymal transition in cervical cancer cells through inactivation of ERK/JNK/p38-MAPK signaling pathways
Abstract
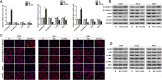
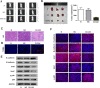
Cervical cancer is an important malignant tumor threatening the physical and mental health of women in the world. As a new calcium activated chloride channel protein, calcium activated chloride channel (CLCA2) plays an important role in tumorigenesis and development. But its role and exact regulatory mechanism in cervical cancer are still unclear. In our study, we found CLCA2 was significantly decreased in cervical cancer cells, and overexpression of CLCA2 inhibited the proliferation, migration and invasion, and promotes apoptosis of cervical cancer cells, and CLCA2 inhibited EMT (Epithelial-mesenchymal transition) through an p38 / JNK / ERK pathway. The results in vivo were consistent with those in vitro. In conclusion, overexpression of CLCA2 inhibited the progression of cervical cancer in vivo and in vitro. This may provide a theoretical basis for CLCA2 as a new indicator of clinical diagnosis and prognosis of cervical cancer or as a potential target of drug therapy.
Keywords: CLCA2; Cervical cancer; Progression; p38.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Buskwofie A, David-West G, Clare CA. A review of cervical Cancer: incidence and disparities [J] J Natl Med Assoc. 2020;112(2):229–232. - PubMed
-
- Jeon YH, Lee HW, Lee YL, et al. Combined E7-dendritic cell-based immunotherapy and human sodium/iodide symporter radioiodine gene therapy with monitoring of antitumor effects by bioluminescent imaging in a mouse model of uterine cervical cancer [J] Cancer Biother Radiopharm. 2011;26(6):671–679. doi: 10.1089/cbr.2011.1081. - DOI - PubMed
-
- Xiao J, Zhou J, Liang L, et al. Sensitivity of ASPP and P-gp to neoadjuvant chemotherapy combined with gene therapy in locally advanced cervical cancer [J] J BUON. 2019;24(3):967–974. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

