Sex-specific changes in triglyceride profiles in liver cirrhosis and hepatitis C virus infection
- PMID: 36280840
- PMCID: PMC9590217
- DOI: 10.1186/s12944-022-01715-w
Sex-specific changes in triglyceride profiles in liver cirrhosis and hepatitis C virus infection
Abstract
Background: Hepatitis C virus (HCV) infection is associated with serum lipid abnormalities, which partly normalize following direct-acting antiviral (DAA) therapy. Here, associations of serum triglycerides (TGs) with viral genotype and markers of liver disease severity were evaluated in patients with chronic HCV. METHODS: The study included the serum of 177 patients with chronic HCV. TGs were quantified by flow injection analysis Fourier transform mass spectrometry. Laboratory values and noninvasive scores for liver fibrosis assessment were determined. The nonparametric Kruskal‒Wallis test, one-way ANOVA, multiple linear regression and Student's t test were used as appropriate. P values were adjusted for multiple comparisons.
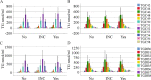
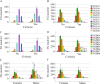
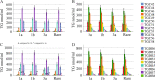
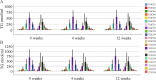
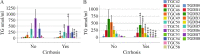
Results: HCV-infected women had lower serum TGs than men, and thus, a sex-specific analysis was performed. None of the 46 TG species analyzed differed in the serum of female patients with and without liver cirrhosis. In contrast, in the serum of male patients with liver cirrhosis, TGs with 53, 56 and 58 carbon atoms and three to eight double bonds were diminished. These polyunsaturated TGs were also low in males with a high fibrosis-4 score. TGs with 7 or 8 double bonds negatively correlated with the model of end-stage liver disease score in males. In addition, TGs with 49, 51 and 53 carbon atoms were reduced in male patients infected with genotype 3a in comparison to genotype 1a. TGs with 56 carbon atoms were lower in genotype 3a-infected males than in genotype 1b-infected males. TGs did not differ in females by genotype. Genotype 3-related changes disappeared at the end of therapy with DAAs. Overall, the levels of serum TGs did not change during DAA therapy in either sex. Consequently, the serum TGs of males with liver cirrhosis were lower than those of males without cirrhosis at the end of therapy. Such a difference was not apparent in females.
Conclusions: The decline in TGs observed only in male patients with liver cirrhosis and male patients infected with genotype 3 illustrates sex-specific changes in lipid metabolism in chronic HCV.
Keywords: Direct acting antivirals; Fibrosis-4 score; Genotype; Liver cirrhosis; Polyunsaturated triglycerides.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance.Int J Mol Sci. 2024 Jul 26;25(15):8161. doi: 10.3390/ijms25158161. Int J Mol Sci. 2024. PMID: 39125730 Free PMC article.
-
Gender-Specific Differences in Serum Sphingomyelin Species in Patients with Hepatitis C Virus Infection-Sphingomyelin Species Are Related to the Model of End-Stage Liver Disease (MELD) Score in Male Patients.Int J Mol Sci. 2023 May 7;24(9):8402. doi: 10.3390/ijms24098402. Int J Mol Sci. 2023. PMID: 37176109 Free PMC article.
-
Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection.Int J Mol Sci. 2022 Aug 29;23(17):9806. doi: 10.3390/ijms23179806. Int J Mol Sci. 2022. PMID: 36077197 Free PMC article.
-
Systematic review: epidemiology and response to direct-acting antiviral therapy in genotype 6 chronic hepatitis C virus infection.Aliment Pharmacol Ther. 2019 Mar;49(5):492-505. doi: 10.1111/apt.15100. Epub 2019 Jan 27. Aliment Pharmacol Ther. 2019. PMID: 30687952
-
Treatment of hepatitis C in children with direct-acting antiviral drugs.Ugeskr Laeger. 2024 Jan 1;186(1):V08230522. doi: 10.61409/V08230522. Ugeskr Laeger. 2024. PMID: 38235776 Review. Danish.
Cited by
-
Cholesterol and Cholesterol-Lowering Medications in COVID-19-An Unresolved Matter.Int J Mol Sci. 2024 Sep 29;25(19):10489. doi: 10.3390/ijms251910489. Int J Mol Sci. 2024. PMID: 39408818 Free PMC article. Review.
-
Lipid Metabolism Disorders as Diagnostic Biosignatures in Sepsis.Infect Dis Rep. 2024 Aug 26;16(5):806-819. doi: 10.3390/idr16050062. Infect Dis Rep. 2024. PMID: 39311203 Free PMC article.
-
Serum Phosphatidylcholine Species 32:0 as a Biomarker for Liver Cirrhosis Pre- and Post-Hepatitis C Virus Clearance.Int J Mol Sci. 2024 Jul 26;25(15):8161. doi: 10.3390/ijms25158161. Int J Mol Sci. 2024. PMID: 39125730 Free PMC article.
-
Hepatitis C Virus Infection Upregulates Plasma Phosphosphingolipids and Endocannabinoids and Downregulates Lysophosphoinositols.Int J Mol Sci. 2023 Jan 11;24(2):1407. doi: 10.3390/ijms24021407. Int J Mol Sci. 2023. PMID: 36674922 Free PMC article.
References
-
- Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, Zeuzem S, Landis CS, Kwo P, Hassan M, et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540–548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

