Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality
- PMID: 36280872
- PMCID: PMC9594899
- DOI: 10.1186/s40104-022-00763-7
Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality
Abstract
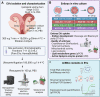
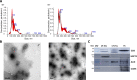
Background: In vitro production of bovine embryos is a well-established technology, but the in vitro culture (IVC) system still warrants improvements, especially regarding embryo quality. This study aimed to evaluate the effect of extracellular vesicles (EVs) isolated from oviductal (OF) and uterine fluid (UF) in sequential IVC on the development and quality of bovine embryos. Zygotes were cultured in SOF supplemented with either BSA or EVs-depleted fetal calf serum (dFCS) in the presence (BSA-EV and dFCS-EV) or absence of EVs from OF (D1 to D4) and UF (D5 to D8), mimicking in vivo conditions. EVs from oviducts (early luteal phase) and uterine horns (mid-luteal phase) from slaughtered heifers were isolated by size exclusion chromatography. Blastocyst rate was recorded on days 7-8 and their quality was assessed based on lipid contents, mitochondrial activity and total cell numbers, as well as survival rate after vitrification. Relative mRNA abundance for lipid metabolism-related transcripts and levels of phosphorylated hormone-sensitive lipase (pHSL) proteins were also determined. Additionally, the expression levels of 383 miRNA in OF- and UF-EVs were assessed by qRT-PCR.
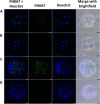
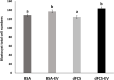
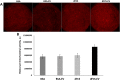
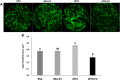
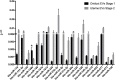
Results: Blastocyst yield was lower (P < 0.05) in BSA treatments compared with dFCS treatments. Survival rates after vitrification/warming were improved in dFCS-EVs (P < 0.05). EVs increased (P < 0.05) blastocysts total cell number in dFCS-EV and BSA-EV compared with respective controls (dFCS and BSA), while lipid content was decreased in dFCS-EV (P < 0.05) and mitochondrial activity did not change (P > 0.05). Lipid metabolism transcripts were affected by EVs and showed interaction with type of protein source in medium (PPARGC1B, LDLR, CD36, FASN and PNPLA2, P < 0.05). Levels of pHSL were lower in dFCS (P < 0.05). Twenty miRNA were differentially expressed between OF- and UF-EVs and only bta-miR-148b was increased in OF-EVs (P < 0.05).
Conclusions: Mimicking physiological conditions using EVs from OF and UF in sequential IVC does not affect embryo development but improves blastocyst quality regarding survival rate after vitrification/warming, total cell number, lipid content, and relative changes in expression of lipid metabolism transcripts and lipase activation. Finally, EVs miRNA contents may contribute to the observed effects.
Keywords: Cattle; Cryopreservation; Embryo development; Exosomes; Lipid metabolism; Oviduct; Uterus; miRNAs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
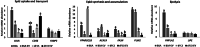

Similar articles
-
Extracellular vesicles-coupled miRNAs from oviduct and uterus modulate signaling pathways related to lipid metabolism and bovine early embryo development.J Anim Sci Biotechnol. 2024 Apr 4;15(1):51. doi: 10.1186/s40104-024-01008-5. J Anim Sci Biotechnol. 2024. PMID: 38570884 Free PMC article.
-
MicroRNA-148b secreted by bovine oviductal extracellular vesicles enhance embryo quality through BPM/TGF-beta pathway.Biol Res. 2024 Mar 23;57(1):11. doi: 10.1186/s40659-024-00488-z. Biol Res. 2024. PMID: 38520036 Free PMC article.
-
Bovine oviductal and uterine fluid support in vitro embryo development.Reprod Fertil Dev. 2018 Jun;30(7):935-945. doi: 10.1071/RD17286. Reprod Fertil Dev. 2018. PMID: 29167013
-
Extracellular vesicles affecting embryo development in vitro: a potential culture medium supplement.Front Pharmacol. 2024 Sep 18;15:1366992. doi: 10.3389/fphar.2024.1366992. eCollection 2024. Front Pharmacol. 2024. PMID: 39359245 Free PMC article. Review.
-
Extracellular Vesicles Mediated Early Embryo-Maternal Interactions.Int J Mol Sci. 2020 Feb 10;21(3):1163. doi: 10.3390/ijms21031163. Int J Mol Sci. 2020. PMID: 32050564 Free PMC article. Review.
Cited by
-
Oviductal extracellular vesicles miRNA cargo varies in response to embryos and their quality.BMC Genomics. 2024 May 27;25(1):520. doi: 10.1186/s12864-024-10429-5. BMC Genomics. 2024. PMID: 38802796 Free PMC article.
-
Factors Affecting the Success of Ovum Pick-Up, In Vitro Production and Cryopreservation of Embryos in Cattle.Animals (Basel). 2025 Jan 25;15(3):344. doi: 10.3390/ani15030344. Animals (Basel). 2025. PMID: 39943114 Free PMC article. Review.
-
Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives.Biology (Basel). 2025 Feb 13;14(2):198. doi: 10.3390/biology14020198. Biology (Basel). 2025. PMID: 40001966 Free PMC article. Review.
-
The Impact of Uterus-Derived Prostaglandins on the Composition of Uterine Fluid During the Period of Conceptus Elongation in Dairy Heifers.Int J Mol Sci. 2025 Feb 20;26(5):1792. doi: 10.3390/ijms26051792. Int J Mol Sci. 2025. PMID: 40076420 Free PMC article.
-
Serum-free spontaneously immortalized bovine oviduct epithelial cell conditioned medium promotes the early development of bovine in vitro fertilized embryos.J Reprod Dev. 2024 Feb 19;70(1):42-48. doi: 10.1262/jrd.2023-031. Epub 2024 Jan 19. J Reprod Dev. 2024. PMID: 38246613 Free PMC article.
References
-
- Rizos D, Lonergan P, Boland MP, Arroyo-García R, Pintado B, de la Fuente J, et al. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod. 2002;66:589–595. doi: 10.1095/biolreprod66.3.589. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

