Neural defensive circuits underlie helping under threat in humans
- PMID: 36281636
- PMCID: PMC9596154
- DOI: 10.7554/eLife.78162
Neural defensive circuits underlie helping under threat in humans
Abstract
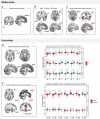
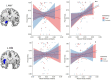
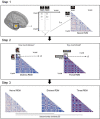
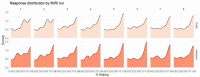
Empathy for others' distress has long been considered the driving force of helping. However, when deciding to help others in danger, one must consider not only their distress, but also the risk to oneself. Whereas the role of self-defense in helping has been overlooked in human research, studies in other animals indicate defensive responses are necessary for the protection of conspecifics. In this pre-registered study (N=49), we demonstrate that human defensive neural circuits are implicated in helping others under threat. Participants underwent fMRI scanning while deciding whether to help another participant avoid aversive electrical shocks, at the risk of also being shocked. We found that higher engagement of neural circuits that coordinate fast escape from self-directed danger (including the insula, PAG, and ACC) facilitated decisions to help others. Importantly, using representational similarity analysis, we found that the strength with which the amygdala and insula uniquely represented the threat to oneself (and not the other's distress) predicted helping. Our findings indicate that in humans, as other mammals, defensive mechanisms play a greater role in helping behavior than previously understood.
Keywords: Brain; Fear; altruism; evolutionary biology; human; neuroscience.
© 2022, Vieira and Olsson.
Conflict of interest statement
JV, AO No competing interests declared
Figures






Similar articles
-
Help or flight? Increased threat imminence promotes defensive helping in humans.Proc Biol Sci. 2020 Aug 26;287(1933):20201473. doi: 10.1098/rspb.2020.1473. Epub 2020 Aug 26. Proc Biol Sci. 2020. PMID: 32842931 Free PMC article.
-
How Human Amygdala and Bed Nucleus of the Stria Terminalis May Drive Distinct Defensive Responses.J Neurosci. 2017 Oct 4;37(40):9645-9656. doi: 10.1523/JNEUROSCI.3830-16.2017. Epub 2017 Sep 11. J Neurosci. 2017. PMID: 28893930 Free PMC article.
-
Amygdala Adaptation and Temporal Dynamics of the Salience Network in Conditioned Fear: A Single-Trial fMRI Study.eNeuro. 2018 Feb 28;5(1):ENEURO.0445-17.2018. doi: 10.1523/ENEURO.0445-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29497705 Free PMC article.
-
The aversive brain system of teleosts: Implications for neuroscience and biological psychiatry.Neurosci Biobehav Rev. 2018 Dec;95:123-135. doi: 10.1016/j.neubiorev.2018.10.001. Epub 2018 Oct 6. Neurosci Biobehav Rev. 2018. PMID: 30300663 Review.
-
Understanding amygdala responsiveness to fearful expressions through the lens of psychopathy and altruism.J Neurosci Res. 2016 Jun;94(6):513-25. doi: 10.1002/jnr.23668. Epub 2015 Sep 14. J Neurosci Res. 2016. PMID: 26366635 Review.
Cited by
-
Acute anxiety during the COVID-19 pandemic was associated with higher levels of everyday altruism.Sci Rep. 2022 Nov 3;12(1):18619. doi: 10.1038/s41598-022-23415-2. Sci Rep. 2022. PMID: 36329157 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

