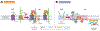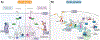Figure 1.. Cellular membrane domain structure and organization.
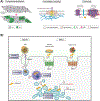
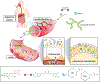
A) Schematic overview of the plasma membrane and several membrane-bound organelles that make up part of the cellular endomembrane system. Membranes are highly dynamic structures that can move intracellularly from the plasma membrane as part of the endocytic pathway. Endocytosis leads to the formation of membrane vesicles, i.e., early endosomes (1), whose lipid bilayer composition resembles that of the plasma membrane. These vesicles mature to form multivesicular structures or late endosomes (2), which can fuse to lysosomes (endolysosome system) (3), or traffic back to the plasma membrane via recycling endosomes (4 and 5). Other key members of the endomembrane system are the nucleus and endoplasmic reticulum (ER). Interestingly, these two organelles work closely together as evidenced by the fact that they share a common continuous membrane bilayer (6). The ER membrane also interacts with the outer mitochondrial membrane (OMM) and a series of proteins, which form a dynamic bridge structure. This dynamic connection is referred to as mitochondria-associated endoplasmic reticulum membranes (MAMs) (7). MAMs domains are indispensable for the execution of a series of cellular functionalities. B) Compartmentalization of proteo-lipid complexes into specialized domains in the plasma membrane. Multiple proteins and lipids assemble in an orchestrated manner to form distinct specialized membrane domains (purple, orange, green and gray regions), thus creating lateral (horizontal axis) heterogeneity in the plasma membrane. Moreover, plasma membrane heterogeneity is displayed across the lipid bilayer vertical axis, resulting in highly organized domains in both the plasma membrane exofacial and cytofacial leaflets. The latter is not disconnected from the former rather they are coupled, thereby facilitating bilayer crosstalk between inner and outer proteolipid components, which is essential for cellular signaling. Liquid ordered phase (Lo) (purple and orange membrane regions) and liquid disordered phase (Ld) (green and gray membrane regions) domains display distinct biochemical, i.e., protein, lipid and carbohydrate, compositions and biophysical, e.g., membrane rigidity, characteristics. The Lo domains are enriched in cholesterol, sphingolipids, glycolipids, and saturated glycerophospholipids as well as lipidated and glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-APs), thus generating lipid raft and raft-like domains. In contrast, the Ld domains, also known as non-rafts, are enriched in polyunsaturated and branched lipids. Additionally, formation, organization and stabilization of these membrane domains are believed to involve cortical actin (cytoskeleton). Finally, these diverse proteolipid domains, shown as purple, orange, green and gray regions, exclude one another, yet they all coexist to form the plasma membrane. Consequently, together these domains form a multifunctional highly complex and dynamic signaling platform. C) MAMs and their functionalities. 1. Lipid synthesis and transfer. MAMs are involved in the synthesis of PS. These structures can generate PS through PSS. Subsequently, PS localized in the ER is transported to the mitochondria and later converted to PE. MAMs can also mediate the trafficking of cholesterol between organelles. In this case, ER-localized caveolin-1 transfers cholesterol from the ER into the mitochondria membrane. 2. Ca2+ trafficking and apoptosis. Another functionality of MAMs is the modulation of programmed cell death. To initiate the apoptosis signaling cascade, IP3R1, localized in the ER membrane, transfers Ca2+ from the ER to mitochondria via VDAC1, which leads to Ca2+ trafficking into the mitochondrial matrix via MCU. The resulting increased mitochondrial Ca2+ levels, triggers the opening of mPTP. Stimulation of Casp8 leads to the activation of downstream caspases and modulation of BCL and PTEN. These proteins, i.e., BCL and PTEN, maintain intraorganellar Ca2+ trafficking. Additionally, SERCA, another MAM-associated protein, removes Ca2+ from the cytosolic space. 3. Mitochondria dynamics. MAM domains are involved in mitochondria fission. The fission by mitochondria involves MFN1/2 and MFN2, which are localized in the outer mitochondrial membrane (OMM). The recruitment of DRP1, a mitochondrial fission protein, to MAMs involves Mff, Fis1 and Syntaxin-17. 4. Autophagy. Under normal conditions, Syntaxin-17 interacts with DRP1 in MAMs. However, in the absence of nutrients, DRP1 is replaced by the pre-autophagosome marker ATG14L, which upregulates mTOR and AKT, and triggers the formation of the autophagosome. 5. ROS and ER stress. MAMs are able to modulate cellular oxidative stress. Under oxidative conditions, p66Shc is phosphorylated, which leads to the relocalization of p66Shc into MAMs. This triggers ROS production, which in turn induces ER stress via IRE1 and PERK. 6. Inflammation. Finally, MAMs play a key role in the modulation of the inflammasome. To initiate this process, NLRP3 traffics from the ER to MAMs where it binds its protein adaptor ASC and TXNIP, which subsequently leads to the formation of the inflammasome. PSS, phosphatidylserine synthase; inositol 1,4,5-trisphosphate receptor type 1; VDAC1, voltage-dependent anion-selective channel protein 1; MCU, mitochondrial calcium uniporter; mPTP, mitochondrial permeability transition pore; CASP8, caspase 8; BCL, B cell lymphoma; PTEN, phosphatase and tensin homolog; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; MFN1, mitofusin 1; MFN2, mitofusin 2; DRP, dynamin-related protein 1; Mff, mitochondrion fission factor; Fis1, fission 1 protein; ATG14L, autophagy-related 14-like; mTOR, mammalian target of rapamycin; AKT, serine/threonine kinase; ROS, reactive oxygen species; p66Shc, member of Src homologous-collagen homologue family of adapter proteins; IRE1, inositol-requiring enzyme 1; PERK, inositol-requiring enzyme 1; NLRP3, pyrin domain-containing 3 protein; ASC, apoptosis-associated speck-like protein; TXNIP, thioredoxin interacting protein.