Ganglioside-Functionalized Nanoparticles for Chimeric Antigen Receptor T-Cell Activation at the Immunological Synapse
- PMID: 36282488
- PMCID: PMC9815837
- DOI: 10.1021/acsnano.2c06516
Ganglioside-Functionalized Nanoparticles for Chimeric Antigen Receptor T-Cell Activation at the Immunological Synapse
Abstract
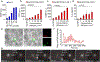
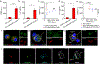
Chimeric Antigen Receptor (CAR) T cell therapy has proven to be an effective strategy against hematological malignancies but persistence and activity against solid tumors must be further improved. One emerging strategy for enhancing efficacy is based on directing CAR T cells to antigen presenting cells (APCs). Activation of CAR T cells at the immunological synapse (IS) formed between APC and T cell is thought to promote strong, persistent antigen-specific T cell-mediated immune responses but requires integration of CAR ligands into the APC/T-cell interface. Here, we demonstrate that CAR ligand functionalized, lipid-coated, biodegradable polymer nanoparticles (NPs) that contain the ganglioside GM3 (GM3-NPs) bind to CD169 (Siglec-1)-expressing APCs and localize to the cell contact site between APCs and CAR T cells upon initiation of cell conjugates. The CD169+ APC/CAR T-cell interface is characterized by a strong optical colocalization of GM3-NPs and CARs, enrichment of F-actin, and recruitment of ZAP-70, indicative of integration of GM3-NPs into a functional IS. Ligands associated with GM3-NPs localized to the APC/T-cell contact site remain accessible to CARs and result in robust T-cell activation. Overall, this work identifies GM3-NPs as a potential antigen delivery platform for active targeting of CD169 expressing APCs and enhancement of CAR T-cell activation at the NP-containing IS.
Keywords: CAR T cell activation; Siglec-1/CD169; antigen presenting cell; biomimetic nanomaterials; ganglioside GM3; lipid-coated polymeric nanoparticle.
Conflict of interest statement
Conflict of Interest
B.M.R. and S.G. hold patents for GM3 functionalized nanoparticles.
Figures





Similar articles
-
Dressing up Nanoparticles: A Membrane Wrap to Induce Formation of the Virological Synapse.ACS Nano. 2015;9(4):4182-92. doi: 10.1021/acsnano.5b00415. Epub 2015 Apr 14. ACS Nano. 2015. PMID: 25853367 Free PMC article.
-
Chimeric antigen receptor T cells targeting the GM3(Neu5Gc) ganglioside.Front Immunol. 2024 Feb 2;15:1331345. doi: 10.3389/fimmu.2024.1331345. eCollection 2024. Front Immunol. 2024. PMID: 38370401 Free PMC article.
-
Immunological Synapse Predicts Effectiveness of Chimeric Antigen Receptor Cells.Mol Ther. 2018 Apr 4;26(4):963-975. doi: 10.1016/j.ymthe.2018.01.020. Epub 2018 Mar 2. Mol Ther. 2018. PMID: 29503199 Free PMC article.
-
The Role of Immunological Synapse in Predicting the Efficacy of Chimeric Antigen Receptor (CAR) Immunotherapy.Cell Commun Signal. 2020 Aug 25;18(1):134. doi: 10.1186/s12964-020-00617-7. Cell Commun Signal. 2020. PMID: 32843053 Free PMC article. Review.
-
Learning from TCR Signaling and Immunological Synapse Assembly to Build New Chimeric Antigen Receptors (CARs).Int J Mol Sci. 2022 Nov 17;23(22):14255. doi: 10.3390/ijms232214255. Int J Mol Sci. 2022. PMID: 36430728 Free PMC article. Review.
Cited by
-
The Ying and Yang of Ganglioside Function in Cancer.Cancers (Basel). 2023 Nov 10;15(22):5362. doi: 10.3390/cancers15225362. Cancers (Basel). 2023. PMID: 38001622 Free PMC article. Review.
-
Exploring CD169+ Macrophages as Key Targets for Vaccination and Therapeutic Interventions.Vaccines (Basel). 2025 Mar 20;13(3):330. doi: 10.3390/vaccines13030330. Vaccines (Basel). 2025. PMID: 40266235 Free PMC article. Review.
-
Advances in bioengineered CAR T/NK cell therapy for glioblastoma: Overcoming immunosuppression and nanotechnology-based strategies for enhanced CAR T/NK cell therapy.Bioeng Transl Med. 2024 Aug 31;10(2):e10716. doi: 10.1002/btm2.10716. eCollection 2025 Mar. Bioeng Transl Med. 2024. PMID: 40060757 Free PMC article. Review.
-
Nanomaterials Mediated Enhancement of CAR-T for HCC: Revolutionizing Immunotherapy Strategies.Int J Nanomedicine. 2025 Jun 13;20:7489-7500. doi: 10.2147/IJN.S527315. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40535838 Free PMC article. Review.
-
Membrane fluidity properties of lipid-coated polylactic acid nanoparticles.Nanoscale. 2024 May 2;16(17):8533-8545. doi: 10.1039/d3nr06464f. Nanoscale. 2024. PMID: 38595322 Free PMC article.
References
-
- Bagley SJ; O’Rourke DM Clinical Investigation of CAR T Cells for Solid Tumors: Lessons Learned and Future Directions. Pharmacol. Ther 2020, 205, 107419. - PubMed
-
- Ma L; Dichwalkar T; Chang JYH; Cossette B; Garafola D; Zhang AQ; Fichter M; Wang C; Liang S; Silva M; Kumari S; Mehta NK; Abraham W; Thai N; Li N; Dane Wittrup K; Irvine DJ Enhanced CAR–T Cell Activity against Solid Tumors by Vaccine Boosting through the Chimeric Receptor. Science 2019, 365, 162–168. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

