Trained immunity is induced in humans after immunization with an adenoviral vector COVID-19 vaccine
- PMID: 36282571
- PMCID: PMC9843058
- DOI: 10.1172/JCI162581
Trained immunity is induced in humans after immunization with an adenoviral vector COVID-19 vaccine
Abstract
BackgroundHeterologous effects of vaccines are mediated by "trained immunity," whereby myeloid cells are metabolically and epigenetically reprogrammed, resulting in heightened responses to subsequent insults. Adenovirus vaccine vector has been reported to induce trained immunity in mice. Therefore, we sought to determine whether the ChAdOx1 nCoV-19 vaccine (AZD1222), which uses an adenoviral vector, could induce trained immunity in vivo in humans.MethodsTen healthy volunteers donated blood on the day before receiving the ChAdOx1 nCoV-19 vaccine and on days 14, 56, and 83 after vaccination. Monocytes were purified from PBMCs, cell phenotype was determined by flow cytometry, expression of metabolic enzymes was quantified by RT-qPCR, and production of cytokines and chemokines in response to stimulation ex vivo was analyzed by multiplex ELISA.ResultsMonocyte frequency and count were increased in peripheral blood up to 3 months after vaccination compared with their own prevaccine controls. Expression of HLA-DR, CD40, and CD80 was enhanced on monocytes for up to 3 months following vaccination. Moreover, monocytes had increased expression of glycolysis-associated enzymes 2 months after vaccination. Upon stimulation ex vivo with unrelated antigens, monocytes produced increased IL-1β, IL-6, IL-10, CXCL1, and MIP-1α and decreased TNF, compared with prevaccine controls. Resting monocytes produced more IFN-γ, IL-18, and MCP-1 up to 3 months after vaccination compared with prevaccine controls.ConclusionThese data provide evidence for the induction of trained immunity following a single dose of the ChAdOx1 nCoV-19 vaccine.FundingThis work was funded by the Health Research Board (EIA-2019-010) and Science Foundation Ireland Strategic Partnership Programme (proposal ID 20/SPP/3685).
Keywords: COVID-19; Cytokines; Glucose metabolism; Monocytes; Vaccines.
Conflict of interest statement
Figures
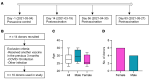
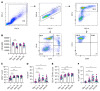
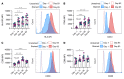

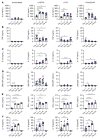

Comment in
- Beyond adaptive immunity: induction of trained immunity by COVID-19 adenoviral vaccines doi: 10.1172/JCI166467
References
-
- Kaufmann E, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2):176–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

