IL-6-targeted therapies to block the cytokine or its receptor drive distinct alterations in T cell function
- PMID: 36282595
- PMCID: PMC9746808
- DOI: 10.1172/jci.insight.159436
IL-6-targeted therapies to block the cytokine or its receptor drive distinct alterations in T cell function
Abstract
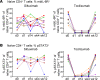
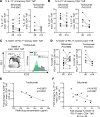
Therapeutics that inhibit IL-6 at different points in its signaling pathway are in clinical use, yet whether the immunological effects of these interventions differ based on their molecular target is unknown. We performed short-term interventions in individuals with type 1 diabetes using anti-IL-6 (siltuximab) or anti-IL-6 receptor (IL-6R; tocilizumab) therapies and investigated the impact of this in vivo blockade on T cell fate and function. Immune outcomes were influenced by the target of the therapeutic intervention (IL-6 versus IL-6R) and by peak drug concentration. Tocilizumab reduced ICOS expression on T follicular helper cell populations and T cell receptor-driven (TCR-driven) STAT3 phosphorylation. Siltuximab reversed resistance to Treg-mediated suppression and increased TCR-driven phosphorylated STAT3 and production of IL-10, IL-21, and IL-27 by T effectors. Together, these findings indicate that the context of IL-6 blockade in vivo drives distinct T cell-intrinsic changes that may influence therapeutic outcomes.
Keywords: Autoimmunity; Cytokines; Immunology; Immunotherapy; T cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

