Maintaining bone health by estrogen therapy in patients with advanced prostate cancer: a narrative review
- PMID: 36283120
- PMCID: PMC9716371
- DOI: 10.1530/EC-22-0182
Maintaining bone health by estrogen therapy in patients with advanced prostate cancer: a narrative review
Abstract
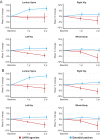
The purpose of androgen deprivation therapy (ADT) in prostate cancer (PCa), using luteinizing hormone-releasing hormone agonists (LHRHa) or gonadotrophin-releasing hormone antagonists, is to suppress the levels of testosterone. Since testosterone is the precursor of estradiol (E2), one of the major undesired effects of ADT is the concomitant loss of E2, causing among others an increased bone turnover and bone loss and an increased risk of osteoporosis and fractures. Therefore, the guidelines for ADT indicate to combine ADT routinely with bone-sparing agents such as bisphosphonates, denosumab or selective estrogen receptor modulators. However, these compounds may have side effects and some require inconvenient parenteral administration. Co-treatment with estrogens is an alternative approach to prevent bone loss and at the same time, to avoid other side effects caused by the loss of estrogens, which is the topic explored in the present narrative review. Estrogens investigated in PCa patients include parenteral or transdermal E2, diethylstilbestrol (DES), and ethinylestradiol (EE) as monotherapy, or high-dose estetrol (HDE4) combined with ADT. Cardiovascular adverse events have been reported with parenteral E2, DES and EE. Encouraging effects on bone parameters have been obtained with transdermal E2 (tE2) and HDE4, in the tE2 development program (PATCH study), and in the LHRHa/HDE4 co-treatment study (PCombi), respectively. Confirmation of the beneficial effects of estrogen therapy with tE2 or HDE4 on bone health in patients with advanced PCa is needed, with special emphasis on bone mass and fracture rate.
Keywords: androgen deprivation therapy (ADT); bone health; estetrol (E4); estrogen therapy (ET); transdermal estradiol (tE2).
Figures


References
-
- Shore ND, Antonarakis ES, Cookson MS, Crawford ED, Morgans AK, Albala DM, Hafron J, Harris RG, Saltzstein D, Brown GA, et al.Optimizing the role of androgen deprivation therapy in advanced prostate cancer: challenges beyond the guidelines. Prostate 202080527–544. (10.1002/pros.23967) - DOI - PMC - PubMed
-
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schroder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU International 20081021531–1538. (10.1111/j.1464-410X.2008.08183.x) - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

