Lack of clinical evidence of antiviral therapy for human monkeypox: A scoping review
- PMID: 36283609
- PMCID: PMC9598785
- DOI: 10.1016/j.jiac.2022.10.009
Lack of clinical evidence of antiviral therapy for human monkeypox: A scoping review
Abstract
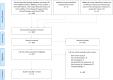
Since May 2022, many human monkeypox cases have been reported from non-endemic countries. This systematic review aimed to evaluate and summarize the existing research on the efficacy and safety of tecovirimat, brincidofovir, and cidofovir for patients with monkeypox. We searched studies that reported the efficacy and adverse events of tecovirimat, brincidofovir, or cidofovir for patients with human monkeypox in several databases including preprint servers. Only five studies were included. The efficacy and adverse events were assessed in only five and four patients, respectively. Regarding tecovirimat, all two patients recovered from monkeypox. One had no adverse event and the other has no description of an adverse event. Regarding brincidofovir, all three patients recovered from monkeypox but all of them had increased alanine transaminase, and one had nausea and abdominal discomfort. There was no study on treatment with cidofovir. Based on past studies and our results, tecovirimat might be the best choice due to ease of administration (oral drug), fewer side effects, and past treatment results for human monkeypox administration. However, very few studies were included in this scoping review. Therefore, further studies are needed to assess their efficacy and safety as possible treatments for human monkeypox.
Keywords: Antiviral agent; Antiviral therapy; Monkeypox; Treatment.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- CDC Monkeypox in the United States. 2021. https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html (accessed June 1, 2022)
-
- Yu J., Raj S.M. Efficacy of three key antiviral drugs used to treat orthopoxvirus infections: a systematic review. Global Biosecurity. 2019;1(1):28. doi: 10.31646/gbio.12. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous