Metformin alleviates bone loss in ovariectomized mice through inhibition of autophagy of osteoclast precursors mediated by E2F1
- PMID: 36284303
- PMCID: PMC9594975
- DOI: 10.1186/s12964-022-00966-5
Metformin alleviates bone loss in ovariectomized mice through inhibition of autophagy of osteoclast precursors mediated by E2F1
Abstract
Background: Postmenopausal bone loss, mainly caused by excessive bone resorption mediated by osteoclasts, has become a global public health burden. Metformin, a hypoglycemic drug, has been reported to have beneficial effects on maintaining bone health. However, the role and underlying mechanism of metformin in ovariectomized (OVX)-induced bone loss is still vague.
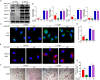
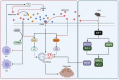
Results: In this study, we demonstrated for the first time that metformin administration alleviated bone loss in postmenopausal women and ovariectomized mice, based on reduced bone resorption markers, increased bone mineral density (BMD) and improvement of bone microstructure. Then, osteoclast precursors administered metformin in vitro and in vivo were collected to examine the differentiation potential and autophagical level. The mechanism was investigated by infection with lentivirus-mediated BNIP3 or E2F1 overexpression. We observed a dramatical inhibition of autophagosome synthesis and osteoclast formation and activity. Treatment with RAPA, an autophagy activator, abrogated the metformin-mediated autophagy downregulation and inhibition of osteoclastogenesis. Additionally, overexpression of E2F1 demonstrated that reduction of OVX-upregulated autophagy mediated by metformin was E2F1 dependent. Mechanistically, metformin-mediated downregulation of E2F1 in ovariectomized mice could downregulate BECN1 and BNIP3 levels, which subsequently perturbed the binding of BECN1 to BCL2. Furthermore, the disconnect between BECN1 and BCL2 was shown by BNIP3 overexpression.
Conclusion: In summary, we demonstrated the effect and underlying mechanism of metformin on OVX-induced bone loss, which could be, at least in part, ascribed to its role in downregulating autophagy during osteoclastogenesis via E2F1-dependent BECN1 and BCL2 downregulation, suggesting that metformin or E2F1 inhibitor is a potential agent against postmenopausal bone loss. Video abstract.
Keywords: Autophagy; BECN1; BNIP3; Bone loss; E2F1; Metformin; Osteoclast precursors.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing financial interests.
Figures







Similar articles
-
TET2 regulates osteoclastogenesis by modulating autophagy in OVX-induced bone loss.Autophagy. 2022 Dec;18(12):2817-2829. doi: 10.1080/15548627.2022.2048432. Epub 2022 Mar 24. Autophagy. 2022. PMID: 35255774 Free PMC article.
-
Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway.Autophagy. 2019 May;15(5):843-870. doi: 10.1080/15548627.2019.1569913. Epub 2019 Jan 27. Autophagy. 2019. PMID: 30653446 Free PMC article.
-
Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis.Mol Med. 2022 Feb 19;28(1):22. doi: 10.1186/s10020-022-00449-w. Mol Med. 2022. PMID: 35183115 Free PMC article.
-
Glycyrrhizic acid suppresses osteoclast differentiation and postmenopausal osteoporosis by modulating the NF-κB, ERK, and JNK signaling pathways.Eur J Pharmacol. 2019 Sep 15;859:172550. doi: 10.1016/j.ejphar.2019.172550. Epub 2019 Jul 16. Eur J Pharmacol. 2019. PMID: 31323222
-
Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis.Osteoporos Int. 2016 Jul;27(7):2335-2344. doi: 10.1007/s00198-016-3496-8. Epub 2016 Jan 25. Osteoporos Int. 2016. PMID: 26809192
Cited by
-
The Potential of Natural Compounds Regulating Autophagy in the Treatment of Osteoporosis.J Inflamm Res. 2023 Dec 8;16:6003-6021. doi: 10.2147/JIR.S437067. eCollection 2023. J Inflamm Res. 2023. PMID: 38088943 Free PMC article. Review.
-
Hypoxia endothelial cells-derived exosomes facilitate diabetic wound healing through improving endothelial cell function and promoting M2 macrophages polarization.Bioact Mater. 2023 Nov 10;33:157-173. doi: 10.1016/j.bioactmat.2023.10.020. eCollection 2024 Mar. Bioact Mater. 2023. PMID: 38034500 Free PMC article.
-
Metformin facilitates osteogenic differentiation of bone marrow stromal cells through AMPK-dependent autophagy: an investigation into the healing of osteoporotic fractures in murine models.J Orthop Surg Res. 2025 Jul 16;20(1):661. doi: 10.1186/s13018-025-06067-6. J Orthop Surg Res. 2025. PMID: 40671012 Free PMC article.
-
Effects of metformin on bone mineral density and bone turnover markers: a systematic review and meta-analysis.BMJ Open. 2023 Jun 23;13(6):e072904. doi: 10.1136/bmjopen-2023-072904. BMJ Open. 2023. PMID: 37355276 Free PMC article.
-
Improvement of osteogenic differentiation potential of placenta-derived mesenchymal stem cells by metformin via AMPK pathway activation.Stem Cell Res Ther. 2024 Nov 13;15(1):417. doi: 10.1186/s13287-024-04014-6. Stem Cell Res Ther. 2024. PMID: 39533406 Free PMC article.
References
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
-
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376. - PubMed
-
- Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

