Drugging KRAS: current perspectives and state-of-art review
- PMID: 36284306
- PMCID: PMC9597994
- DOI: 10.1186/s13045-022-01375-4
Drugging KRAS: current perspectives and state-of-art review
Abstract
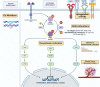
After decades of efforts, we have recently made progress into targeting KRAS mutations in several malignancies. Known as the 'holy grail' of targeted cancer therapies, KRAS is the most frequently mutated oncogene in human malignancies. Under normal conditions, KRAS shuttles between the GDP-bound 'off' state and the GTP-bound 'on' state. Mutant KRAS is constitutively activated and leads to persistent downstream signaling and oncogenesis. In 2013, improved understanding of KRAS biology and newer drug designing technologies led to the crucial discovery of a cysteine drug-binding pocket in GDP-bound mutant KRAS G12C protein. Covalent inhibitors that block mutant KRAS G12C were successfully developed and sotorasib was the first KRAS G12C inhibitor to be approved, with several more in the pipeline. Simultaneously, effects of KRAS mutations on tumour microenvironment were also discovered, partly owing to the universal use of immune checkpoint inhibitors. In this review, we discuss the discovery, biology, and function of KRAS in human malignancies. We also discuss the relationship between KRAS mutations and the tumour microenvironment, and therapeutic strategies to target KRAS. Finally, we review the current clinical evidence and ongoing clinical trials of novel agents targeting KRAS and shine light on resistance pathways known so far.
© 2022. The Author(s).
Conflict of interest statement
K.P. reports advisory board fees from Guardant Health and Jazz Pharmaceuticals. G.L.B. reports personal fees from AstraZeneca, Astellas, travel and conference expenses from Janssen. S.V.L. reports advisory board/consultant fees from Amgen, AstraZeneca, Bayer, Beigene, Blueprint, Boehringer-Ingelheim, Bristol-Myers Squibb, Catalyst, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech/Roche, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Lilly, Merck/MSD, Novartis, Regeneron, Sanofi, Takeda, Turning Point Therapeutics and research grant (to institution) from Alkermes, Blueprint, Bristol-Myers Squibb, Elevation Oncology, Genentech, Gilead, Merck, Merus, Nuvalent, Pfizer, RAPT, Turning Point Therapeutics. A.F. reports consulting fees from Amgen, Astrazeneca, Roche, Astellas, Takeda, Bristol-Myers Squibb, Merck Sharpe Dohme, Pfizer, Merck, Novartis, Janssen. A.A. reports advisory board fees from Merck Sharpe Dohme, Roche, Takeda, Pfizer, Bristol-Myers Squibb, AstraZeneca, Eli-Lilly; speaker’s bureau fees from Eli-Lilly, AstraZeneca, Amgen; SA consulting or advisory fees from Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Roche, Novartis, MSD Oncology, Pfizer, Takeda, AstraZeneca; expert testimony fees from Roche, AstraZeneca, Bristol Myers Squibb; travels, accommodations, expenses from Bristol Myers Squibb, AstraZeneca, Amgen. V.S. reports research funding/grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty Ltd.; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-Pharm US; and other relationship with Medscape.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020 et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Harvey JJ. An unidentified virus which causes the rapid production of tumours in mice. Nature. 1964;204:1104–1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous