B3GALT4 remodels the tumor microenvironment through GD2-mediated lipid raft formation and the c-met/AKT/mTOR/IRF-1 axis in neuroblastoma
- PMID: 36284313
- PMCID: PMC9594894
- DOI: 10.1186/s13046-022-02523-x
B3GALT4 remodels the tumor microenvironment through GD2-mediated lipid raft formation and the c-met/AKT/mTOR/IRF-1 axis in neuroblastoma
Abstract
Background: Beta-1,3-galactosyltransferase-4 (B3GALT4) plays a critical regulatory role in tumor biology. However, the role of B3GALT4 in modulating the tumor microenvironment (TME) of neuroblastoma (NB) remains unknown.
Methods: Public datasets and clinical NB samples were collected to evaluate the expression and clinical significance of GD2 and B3GALT4 in NB patients. CCK-8, colony formation, and transwell assays and experiments in tumor-bearing mouse models were conducted to investigate the function of B3GALT4. Flow cytometry, ELISA, immunohistochemistry, immunofluorescence, western blotting, and chemotaxis assays were conducted to ascertain the immunomodulatory mechanism of B3GALT4. The combined therapeutic effect of the lipid raft inhibitor MβCD and anti-GD2 mAb was validated in a murine model of NB.
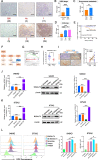
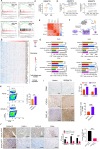
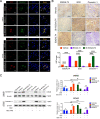
Results: GD2 was overexpressed in NB tissues and high expression of GD2 was associated with poor prognosis in NB patients. B3GALT4 was downregulated in NB tissues, and low expression of B3GALT4 indicated poor prognosis in NB patients. Silencing B3GALT4 significantly enhanced tumor progression both in vitro and in vivo. Meanwhile, the overexpression of B3GALT4 increased the recruitment of CD8+ T lymphocytes via the chemokines CXCL9 and CXCL10. Additionally, B3GALT4 regulated NB-cell GD2 expression and lipid raft formation. Mechanistically, B3GALT4 regulated the expression of CXCL9 and CXCL10 via the c-Met signaling in the lipid rafts and the downstream AKT/mTOR/IRF-1 pathway. The lipid raft inhibitor, MβCD, attenuated B3GALT4 deficiency-induced tumor progression and immune evasion. Last, MβCD combined with anti-GD2 mAb treatment significantly enhanced the antitumor effect and the infiltration of CD8+ T cells.
Conclusions: Upregulation of B3GALT4 promotes the secretion of CXCL9 and CXCL10 to recruit CD8+ T lymphocytes via the GD2-mediated lipid rafts and the c-Met/AKT/mTOR/IRF-1 pathway. Moreover, lipid raft inhibitors may enhance the efficacy of anti-GD2 immunotherapy for NB.
Keywords: Beta-1,3-galactosyltransferase-4; CD8+ T-cell chemokine; Ganglioside GD2; Immunotherapy; Lipid raft; Neuroblastoma.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Alexander F. Neuroblastoma. Urol Clin North Am. 2000;27(3):383–392. - PubMed
-
- Layer JP, Kronmuller MT, Quast T, van den Boorn-Konijnenberg D, Effern M, Hinze D, et al. Amplification of N-Myc is associated with a T-cell-poor microenvironment in metastatic neuroblastoma restraining interferon pathway activity and chemokine expression. Oncoimmunology. 2017;6(6):e1320626. - PMC - PubMed
-
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

