Effects of dabigatran versus warfarin on 2-year cognitive outcomes in old patients with atrial fibrillation: results from the GIRAF randomized clinical trial
- PMID: 36284318
- PMCID: PMC9598018
- DOI: 10.1186/s12916-022-02563-2
Effects of dabigatran versus warfarin on 2-year cognitive outcomes in old patients with atrial fibrillation: results from the GIRAF randomized clinical trial
Abstract
Background: Observational studies support a role for oral anticoagulation to reduce the risk of dementia in atrial fibrillation patients, but conclusive data are lacking. Since dabigatran offers a more stable anticoagulation, we hypothesized it would reduce cognitive decline when compared to warfarin in old patients with atrial fibrillation.
Methods: The GIRAF trial was a 24-month, randomized, parallel-group, controlled, open-label, hypothesis generating trial. The trial was done in six centers including a geriatric care unit, secondary and tertiary care cardiology hospitals in São Paulo, Brazil. We included patients aged ≥ 70 years and CHA2DS2-VASc score > 1. The primary endpoint was the absolute difference in cognitive performance at 2 years. Patients were assigned 1:1 to take dabigatran (110 or 150 mg twice daily) or warfarin, controlled by INR and followed for 24 months. Patients were evaluated at baseline and at 2 years with a comprehensive and thorough cognitive evaluation protocol of tests for different cognitive domains including the Montreal Cognitive Assessment (MoCA), Mini-Mental State Exam (MMSE), a composite neuropsychological test battery (NTB), and computer-generated tests (CGNT).
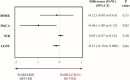
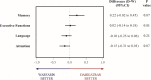
Results: Between 2014 and 2019, 5523 participants were screened and 200 were assigned to dabigatran (N = 99) or warfarin (N = 101) treatment. After adjustment for age, log of years of education, and raw baseline score, the difference between the mean change from baseline in the dabigatran group minus warfarin group was - 0.12 for MMSE (95% confidence interval [CI] - 0.88 to 0.63; P = 0.75), 0.05 (95% CI - 0.07 to 0.18; P = 0.40) for NTB, - 0.15 (95% CI - 0.30 to 0.01; P = 0.06) for CGNT, and - 0.96 (95% CI - 1.80 to 0.13; P = 0.02) for MoCA, with higher values suggesting less cognitive decline in the warfarin group.
Conclusions: For elderly patients with atrial fibrillation, and without cognitive compromise at baseline that did not have stroke and were adequately treated with warfarin (TTR of 70%) or dabigatran for 2 years, there was no statistical difference at 5% significance level in any of the cognitive outcomes after adjusting for multiple comparisons.
Trial registration: Cognitive Impairment Related to Atrial Fibrillation Prevention Trial (GIRAF), NCT01994265 .
Keywords: Atrial fibrillation; Cognitive scores; Dabigatran; Dementia; Elderly; Warfarin.
© 2022. The Author(s).
Conflict of interest statement
BC received consulting fee from Bayer, SERVIER, and Elsevier; DKA and RRS performed the neuropsychological tests through a grant from Boehringer Ingelheim do Brasil; PCY perfomed nursing care of the patients along the study period through a grant from Boehringer Ingelheim do Brasil; MGM performed nursing care of the patients along the study period through a grant from Boehringer Ingelheim do Brasil; DC received consulting fee from Bayer, Daiichi-Sankyo, Janssen, and received research grant from Stago; FAMC received honoraria for lectures from Bayer; PC has participation as principal investigator in clinical trials for Novo Nordisk and participation in advisory boards for Ach, Biogen, EMS, Nutricia, Roche, and developed material for continuous medical education and participation as speaker in symposia sponsored by Ach, Nutricia, Libbs, Roche, Sandoz, Torrent, and Zodiac. ACR, RN, MCE-R, CAMT, IRMER, DMG, FAB-J, AFP, and SMB have no disclosures.
Figures

References
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

