Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis
- PMID: 36284334
- PMCID: PMC9594889
- DOI: 10.1186/s13054-022-04140-8
Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis
Abstract
Background: The enteral route is commonly utilised to support the nutritional requirements of critically ill patients. However, there is paucity of data guiding clinicians regarding the appropriate method of delivering the prescribed dose. Continuous enteral feeding is commonly used; however, a bolus or intermittent method of administration may provide several advantages such as minimising interruptions. The purpose of this meta-analysis is to compare a continuous versus an intermittent or bolus enteral nutrition administration method.
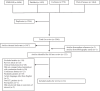
Methods: A systematic review and meta-analysis were performed with studies identified from the PubMed, EMBASE, Cochrane Library and Web of Science databases. Studies were included if they compared a continuous with either an intermittent or bolus administration method of enteral nutrition in adult patients admitted to the intensive care unit. Study quality was assessed using the PEDro and Newcastle-Ottawa scoring systems. Review Manager was used for performing the random-effects meta-analysis on the outcomes of mortality, constipation, diarrhoea, increased gastric residuals, pneumonia, and bacterial colonisation.
Results: A total of 5546 articles were identified, and 133 were included for full text review. Fourteen were included in the final analysis. There was an increased risk of constipation with patients receiving continuous enteral nutrition (relative risk 2.24, 95% confidence interval 1.01-4.97, p = 0.05). No difference was identified in other outcome measures. No appreciable bias was identified.
Conclusion: The current meta-analysis has not identified any clinically relevant difference in most outcome measures relevant to the care of critically ill patients. However, there is a paucity of high-quality randomised controlled clinical trials to guide this decision. Therefore, clinicians may consider either dosing regimen in the context of the patient's care requirements.
Keywords: Enteral nutrition; Gastric residuals; Intensive care unit.
© 2022. The Author(s).
Conflict of interest statement
No authors have any conflicts of interest to declare.
Figures

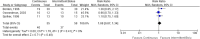
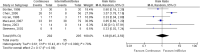

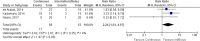

References
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) J Parenter Enter Nutr. 2016;40:159–211. - PubMed
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. - PubMed
-
- Patel JJ, Kozeniecki M, Peppard WJ, Peppard SR, Zellner-Jones S, Graf J, et al. Phase 3 pilot randomized controlled trial comparing early trophic enteral nutrition with "no enteral nutrition" in mechanically ventilated patients with septic shock. J Parenter Enter Nutr. 2020;44:866–873. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

