Severe Acute Respiratory Infection-Preparedness: Protocol for a Multicenter Prospective Cohort Study of Viral Respiratory Infections
- PMID: 36284548
- PMCID: PMC9586923
- DOI: 10.1097/CCE.0000000000000773
Severe Acute Respiratory Infection-Preparedness: Protocol for a Multicenter Prospective Cohort Study of Viral Respiratory Infections
Abstract
Respiratory virus infections cause significant morbidity and mortality ranging from mild uncomplicated acute respiratory illness to severe complications, such as acute respiratory distress syndrome, multiple organ failure, and death during epidemics and pandemics. We present a protocol to systematically study patients with severe acute respiratory infection (SARI), including severe acute respiratory syndrome coronavirus 2, due to respiratory viral pathogens to evaluate the natural history, prognostic biomarkers, and characteristics, including hospital stress, associated with clinical outcomes and severity.
Design: Prospective cohort study.
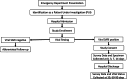
Setting: Multicenter cohort of patients admitted to an acute care ward or ICU from at least 15 hospitals representing diverse geographic regions across the United States.
Patients: Patients with SARI caused by infection with respiratory viruses that can cause outbreaks, epidemics, and pandemics.
Interventions: None.
Measurements and main results: Measurements include patient demographics, signs, symptoms, and medications; microbiology, imaging, and associated tests; mechanical ventilation, hospital procedures, and other interventions; and clinical outcomes and hospital stress, with specimens collected on days 0, 3, and 7-14 after enrollment and at discharge. The primary outcome measure is the number of consecutive days alive and free of mechanical ventilation (VFD) in the first 30 days after hospital admission. Important secondary outcomes include organ failure-free days before acute kidney injury, shock, hepatic failure, disseminated intravascular coagulation, 28-day mortality, adaptive immunity, as well as immunologic and microbiologic outcomes.
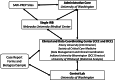
Conclusions: SARI-Preparedness is a multicenter study under the collaboration of the Society of Critical Care Medicine Discovery, Resilience Intelligence Network, and National Emerging Special Pathogen Training and Education Center, which seeks to improve understanding of prognostic factors associated with worse outcomes and increased resource utilization. This can lead to interventions to mitigate the clinical impact of respiratory virus infections associated with SARI.
Keywords: COVID-19; clinical research networks; hospitalized patients; observational study; severe acute respiratory syndrome coronavirus 2.
Conflict of interest statement
Dr. Anesi receives research funding from the Agency for Healthcare Research and Quality and reports payments for authoring COVID-19 chapters for UpToDate and for expert witness consulting. Dr. Segal receives grant funding from the National Institutes of Health (Method to Extend Research in Time R37 CA244775) paid to institution, and Dr. Wyles receives funding from Gilead Sciences paid to institution (ended August 31, 2021). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- World Health Organization. COVID-19 Weekly Epidemiological Update Edition 54. Published August 24, 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Accessed. October 29, 2021.
Grants and funding
LinkOut - more resources
Full Text Sources