The efficacy and safety of thalidomide in the treatment of refractory Crohn's disease in adults: a double-center, double-blind, randomized-controlled trial
- PMID: 36284737
- PMCID: PMC9583847
- DOI: 10.1093/gastro/goac052
The efficacy and safety of thalidomide in the treatment of refractory Crohn's disease in adults: a double-center, double-blind, randomized-controlled trial
Abstract
Background: Thalidomide is applied in therapy for refractory Crohn's disease (CD) in adults, but systematic and rigorous clinical evidence is scant. The aim was to provide theoretical references for the efficacy of thalidomide in the therapy for refractory CD in adults.
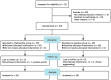
Methods: A double-center, double-blind, placebo-controlled, randomized clinical trial of refractory CD in adults in two inflammatory bowel disease centers in China. In the double-blind trial, patients were randomly assigned to 100 mg of thalidomide or placebo daily for 8 weeks. The primary outcome was considered as the clinical remission rate calculated based on the Crohn's disease activity index at the eighth week following thalidomide or placebo treatment. In open label, non-response to placebo was additionally treated with 8 weeks of thalidomide; all responders were continuously treated with thalidomide until the 48th week.
Results: Twenty-five patients were randomly assigned to each group. At the eighth week, the clinical remission rate in the thalidomide group was significantly higher than that in the placebo group (68.0% [17/25] vs 16.0% [4/25]; relative risk, 4.2; 95% confidence interval, 1.8-10.9, P < 0.001). After a 48-week follow-up, the continuous treatment rate of thalidomide was 46.3% (19/41). Adverse events during the whole process were reported in 58.5% of patients, mainly involving drowsiness, rash, and peripheral neuropathy that were mild and tolerable.
Conclusion: Thalidomide can be used in the induction and maintenance therapy of refractory CD in adults. And it could be one of the treatment options for refractory CD.
Keywords: Crohn’s disease; clinical remission; refractory; thalidomide.
© The Author(s) 2022. Published by Oxford University Press and Sixth Affiliated Hospital of Sun Yat-sen University.
Figures
References
-
- El-Matary W, Moroz SP, Bernstein CN.. Inflammatory bowel disease in children of Manitoba: 30 years' experience of a tertiary center. J Pediatr Gastroenterol Nutr 2014;59:763–6. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. - PubMed
-
- Gisbert JP, Panes J.. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol 2009;104:760–7. - PubMed
-
- Billioud V, Sandborn WJ, Peyrin-Biroulet L.. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol 2011;106:674–84. - PubMed
LinkOut - more resources
Full Text Sources