Targeting the high affinity receptor, FcγRI, in autoimmune disease, neuropathy, and cancer
- PMID: 36284837
- PMCID: PMC9585681
- DOI: 10.1093/immadv/ltac011
Targeting the high affinity receptor, FcγRI, in autoimmune disease, neuropathy, and cancer
Abstract
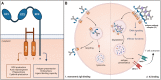
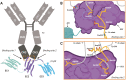
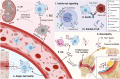
The Fc gamma receptor I (FcγRI or CD64) is the only human Fc receptor with a high affinity for monomeric IgG. It plays a crucial role in immunity, as it mediates cellular effector functions of antibodies including phagocytosis, antigen presentation, and cytokine production. FcγRI is constitutively saturated with monomeric IgG and this feeds the dogma that it has no role in immune responses. However, recent findings have implicated a role for FcγRI in various autoimmune disorders, neuropathies, and antibody therapy in tumor models. By a process known as 'inside-out' signaling, stimulation of myeloid cells with cytokines such as tumor necrosis factor alpha (TNF-α) and interferon-gamma (IFN-γ) enhances FcγRI binding to immune complexes (ICs), including antibody-opsonized pathogens or tumor cells. This review focuses on the current knowledge on interaction of FcγRI with IgG and ICs and the effect of inside-out signaling on FcγRI functioning. Additionally, this review will address potential clinical applications of targeting FcγRI, and the tools that can be used to overcome IC-mediated autoimmune diseases on the one hand, and to enhance antibody-based anti-cancer therapy on the other.
Keywords: CD64; autoimmunity; cancer; inside-out signaling; neuropathy; therapeutic antibodies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
