DNAM1 and TIGIT balance the T cell response, with low T cell TIGIT expression corresponding to inflammation in psoriatic disease
- PMID: 36284900
- PMCID: PMC9585685
- DOI: 10.1093/immadv/ltaa004
DNAM1 and TIGIT balance the T cell response, with low T cell TIGIT expression corresponding to inflammation in psoriatic disease
Abstract
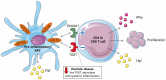
Objectives: Signals at the contact site of antigen-presenting cells (APCs) and T cells help orchestrate the adaptive immune response. CD155 on APCs can interact with the stimulatory receptor DNAM1 or inhibitory receptor TIGIT on T cells. The CD155/DNAM1/TIGIT axis is under extensive investigation as immunotherapy target in inflammatory diseases including cancer, chronic infection and autoimmune diseases. We investigated a possible role for CD155/DNAM1/TIGIT signaling in psoriatic disease.
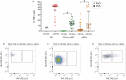
Methods: By flow cytometry, we analyzed peripheral blood mononuclear cells of patients with psoriasis (n = 20) or psoriatic arthritis (n = 21), and healthy individuals (n = 7). We measured CD155, TIGIT, and DNAM1 expression on leukocyte subsets and compared activation-induced cytokine production between CD155-positive and CD155-negative APCs. We assessed the effects of TIGIT and DNAM1 blockade on T cell activation, and related the expression of CD155/DNAM1/TIGIT axis molecules to measures of disease activity.
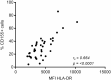
Results: High CD155 expression associates with tumor necrosis factor (TNF) production in myeloid and plasmacytoid dendritic cells (DC). In CD1c+ myeloid DC, activation-induced CD155 expression associates with increased HLA-DR expression. CD8 T cells - but not CD4 T cells - express high levels of TIGIT. DNAM1 blockade decreases T cell pro-inflammatory cytokine production, while TIGIT blockade increased T cell proliferation. Finally, T cell TIGIT expression shows an inverse correlation with inflammation biomarkers in psoriatic disease.
Conclusion: CD155 is increased on pro-inflammatory APCs, while the receptors DNAM1 and TIGIT expressed on T cells balance the inflammatory response by T cells. In psoriatic disease, low TIGIT expression on T cells is associated with systemic inflammation.
Keywords: CD155; DNAM1; TIGIT; inflammation; psoriatic disease.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
