Radiomics as an emerging tool in the management of brain metastases
- PMID: 36284932
- PMCID: PMC9583687
- DOI: 10.1093/noajnl/vdac141
Radiomics as an emerging tool in the management of brain metastases
Abstract
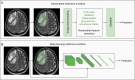
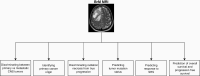
Brain metastases (BM) are associated with significant morbidity and mortality in patients with advanced cancer. Despite significant advances in surgical, radiation, and systemic therapy in recent years, the median overall survival of patients with BM is less than 1 year. The acquisition of medical images, such as computed tomography (CT) and magnetic resonance imaging (MRI), is critical for the diagnosis and stratification of patients to appropriate treatments. Radiomic analyses have the potential to improve the standard of care for patients with BM by applying artificial intelligence (AI) with already acquired medical images to predict clinical outcomes and direct the personalized care of BM patients. Herein, we outline the existing literature applying radiomics for the clinical management of BM. This includes predicting patient response to radiotherapy and identifying radiation necrosis, performing virtual biopsies to predict tumor mutation status, and determining the cancer of origin in brain tumors identified via imaging. With further development, radiomics has the potential to aid in BM patient stratification while circumventing the need for invasive tissue sampling, particularly for patients not eligible for surgical resection.
Keywords: artificial intelligence; brain metastases; radiology; radiomics.
© The Author(s) 2022. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures
References
-
- Achrol AS, Rennert RC, Anders C, et al. . Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. - PubMed
-
- Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249(10):1357–1369. - PubMed
-
- Suh JH, Kotecha R, Chao ST, et al. . Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. - PubMed
-
- Gaspar L, Scott C, Rotman M, et al. . Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
