Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages
- PMID: 36285103
- PMCID: PMC9547697
- DOI: 10.21037/jss-22-17
Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages
Abstract
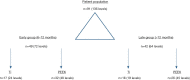
Background: Compare fusion at two independent timepoints (early and late) between 3D-printed titanium (Ti) and polyetheretherketone (PEEK) cages in patients undergoing standalone lateral lumbar interbody fusion (SA-LLIF). We hypothesized that 3D-printed Ti cages show higher fusion rates at an early timepoint compared to PEEK.
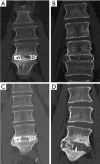
Methods: A retrospective study of patients undergoing SA-LLIF with 3D-printed Ti cages and PEEK cages between 11/2016 and 01/2020 at a single academic institution was done. Fusion was assessed for each treated level using multiplanar reconstructed computed tomography (CT) scans. Presence of fully bridged interbody trabecular bone or continuous bone centered in the cage was considered as fusion.
Results: In total, 91 patients (136 levels) were included in the final analysis, 49 patients (72 levels) in the early group and 42 patients (64 levels) in the late group. CT scans were performed on average 8.2±1.8 months postoperatively for the early group and 18.9±7.7 months for the late group. In the early group, fusion was significantly higher for 3D-printed Ti cages compared to PEEK cages (95.8% versus 62.5%; P=0.002), whereas in the late group no significant difference was seen (94.7% versus 80.0%; P=0.258).
Conclusions: In SA-LLIF, porous 3D-printed Ti cages showed significantly higher fusion rates at an early timepoint compared to PEEK. However, the difference in fusion rates between 3D-printed Ti cages and PEEK cages was found not to be significantly different at a later timepoint in another patient group. This might support the assumption that 3D-printed Ti cages with a porous architecture are more osteoconductive compared to PEEK and tend to fuse earlier.
Keywords: Lateral lumbar interbody fusion (LLIF); fusion; lumbar fusion; standalone; titanium (Ti).
2022 Journal of Spine Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-17/coif). AAS declares financial interests: Ortho Development Corp, Vestia Ventures MiRUS Investment LLC, ISPH II LLC, ISPH 3 LLC, VBros Venture Partners X Centinel Spine, Clariance Inc., Kuros Bioscience AG, Medical Device Business Services Inc., DePuy Synthes Products Inc., Medical Device Business Services Inc. and Spinal Kinetcs Inc. FPC declares financial interests: NuVasive Inc., Bonovo Orthopedics Inc., Healthpoint Capital Partners LP, ISPH II LLC, Ivy Healthcare Capital Partners LLC, Medical Device Partners II LLC, Medical Device Partners III LLC, Orthobond Corporation, Spine Biopharma LLC, Tissue Differentiation Intelligence LLC, VBVP VI LLC, Woven Orthopedics Technologies, 4Web Medical/4Web Inc., Spine Biopharma LLC and Beatrice & Samuel A. Seaver Foundation. FPG declares financial interests: NuVasive Inc., Ortho Development Corp, Zimmer Biomet Holdings INC, Bonovo Orthopedics Inc., Liventa Bioscience (AF Cell Medical), Paradigm Spine LLC, Healthpoint Capital Partners LP, Alphatec Holdings LLC, LANX Inc., Centinel Spine Inc. (fka Raymedica LLC), Tissue Differentiation Intelligence LLC, Spine Kinetics Inc., DePuy Synthes Spine and NuVasive Inc. APH declares financial interests: 4Web Medical, NuVasive Inc. and Kuros Bioscience B.V. The other authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Evaluation of cage subsidence in standalone lateral lumbar interbody fusion: novel 3D-printed titanium versus polyetheretherketone (PEEK) cage.Eur Spine J. 2021 Aug;30(8):2377-2384. doi: 10.1007/s00586-021-06912-2. Epub 2021 Jul 2. Eur Spine J. 2021. PMID: 34215921
-
3D-printed porous titanium versus polyetheretherketone cages in lateral lumbar interbody fusion: a systematic review and meta-analysis of subsidence.Front Med (Lausanne). 2024 Dec 18;11:1389533. doi: 10.3389/fmed.2024.1389533. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39744537 Free PMC article.
-
Early Outcomes of Three-Dimensional-Printed Porous Titanium versus Polyetheretherketone Cage Implantation for Stand-Alone Lateral Lumbar Interbody Fusion in the Treatment of Symptomatic Adjacent Segment Degeneration.World Neurosurg. 2022 Jun;162:e14-e20. doi: 10.1016/j.wneu.2021.11.122. Epub 2021 Dec 1. World Neurosurg. 2022. PMID: 34863938
-
Do Three-Dimensional Printed Porous Titanium Relative to Polyetheretherketone Interbody Cages Reduce Complications and Revisions after Transforaminal Lumbar Interbody Fusion?Global Spine J. 2025 May 19:21925682251339998. doi: 10.1177/21925682251339998. Online ahead of print. Global Spine J. 2025. PMID: 40387406 Free PMC article.
-
Titanium-coated PEEK Versus Uncoated PEEK Cages in Lumbar Interbody Fusion: A Systematic Review and Meta-analysis of Randomized Controlled Trial.Clin Spine Surg. 2023 Jun 1;36(5):198-209. doi: 10.1097/BSD.0000000000001378. Epub 2022 Aug 24. Clin Spine Surg. 2023. PMID: 35994033 Free PMC article.
Cited by
-
Fundamentals of Mechanobiology and Potential Applications in Spinal Fusion.Int J Spine Surg. 2023 Dec 27;17(S3):S61-S74. doi: 10.14444/8562. Int J Spine Surg. 2023. PMID: 38135446 Free PMC article.
-
Comparison between Three-Dimensional Printed Titanium and PEEK Cages for Cervical and Lumbar Interbody Fusion: A Prospective Controlled Trial.Orthop Surg. 2023 Nov;15(11):2889-2900. doi: 10.1111/os.13896. Epub 2023 Sep 28. Orthop Surg. 2023. PMID: 37771127 Free PMC article. Clinical Trial.
-
3D-printed titanium alloy cage in anterior and lateral lumbar interbody fusion for degenerative lumbar spine disease.J Spine Surg. 2024 Mar 20;10(1):22-29. doi: 10.21037/jss-23-120. Epub 2024 Mar 14. J Spine Surg. 2024. PMID: 38567003 Free PMC article.
-
Impact of Habitual Flexion on Bone Formation After Spinal Fusion Surgery: An In Silico Study.JOR Spine. 2025 Jul 14;8(3):e70075. doi: 10.1002/jsp2.70075. eCollection 2025 Sep. JOR Spine. 2025. PMID: 40662111 Free PMC article.
-
Lumbar lateral interbody fusion: step-by-step surgical technique and clinical experience.J Spine Surg. 2023 Sep 22;9(3):294-305. doi: 10.21037/jss-23-54. Epub 2023 Sep 12. J Spine Surg. 2023. PMID: 37841793 Free PMC article.
References
-
- Park P, Than KD, Mummaneni PV, et al. Factors affecting approach selection for minimally invasive versus open surgery in the treatment of adult spinal deformity: analysis of a prospective, nonrandomized multicenter study. J Neurosurg Spine 2020. [Epub ahead of print]. doi: .10.3171/2020.4.SPINE20169 - DOI - PubMed
LinkOut - more resources
Full Text Sources
