Genomic and functional analysis of phage-mediated horizontal gene transfer in Pseudomonas syringae on the plant surface
- PMID: 36285389
- PMCID: PMC10107160
- DOI: 10.1111/nph.18573
Genomic and functional analysis of phage-mediated horizontal gene transfer in Pseudomonas syringae on the plant surface
Abstract
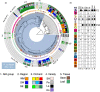
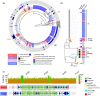
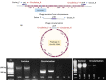
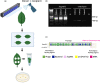
Many strains of Pseudomonas colonise plant surfaces, including the cherry canker pathogens, Pseudomonas syringae pathovars syringae and morsprunorum. We have examined the genomic diversity of P. syringae in the cherry phyllosphere and focused on the role of prophages in transfer of genes encoding Type 3 secreted effector (T3SE) proteins contributing to the evolution of virulence. Phylogenomic analysis was carried out on epiphytic pseudomonads in the UK orchards. Significant differences in epiphytic populations occurred between regions. Nonpathogenic strains were found to contain reservoirs of T3SE genes. Members of P. syringae phylogroups 4 and 10 were identified for the first time from Prunus. Using bioinformatics, we explored the presence of the gene encoding T3SE HopAR1 within related prophage sequences in diverse P. syringae strains including cherry epiphytes and pathogens. Results indicated that horizontal gene transfer (HGT) of this effector between phylogroups may have involved phage. Prophages containing hopAR1 were demonstrated to excise, circularise and transfer the gene on the leaf surface. The phyllosphere provides a dynamic environment for prophage-mediated gene exchange and the potential for the emergence of new more virulent pathotypes. Our results suggest that genome-based epidemiological surveillance of environmental populations will allow the timely application of control measures to prevent damaging diseases.
Keywords: Pseudomonas syringae; bacterial evolution; genomic diversity; horizontal gene transfer (HGT); phyllosphere; prophage.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

