Thermoelectric Silver-Based Chalcogenides
- PMID: 36285805
- PMCID: PMC9799025
- DOI: 10.1002/advs.202204624
Thermoelectric Silver-Based Chalcogenides
Abstract
Heat is abundantly available from various sources including solar irradiation, geothermal energy, industrial processes, automobile exhausts, and from the human body and other living beings. However, these heat sources are often overlooked despite their abundance, and their potential applications remain underdeveloped. In recent years, important progress has been made in the development of high-performance thermoelectric materials, which have been extensively studied at medium and high temperatures, but less so at near room temperature. Silver-based chalcogenides have gained much attention as near room temperature thermoelectric materials, and they are anticipated to catalyze tremendous growth in energy harvesting for advancing internet of things appliances, self-powered wearable medical systems, and self-powered wearable intelligent devices. This review encompasses the recent advancements of thermoelectric silver-based chalcogenides including binary and multinary compounds, as well as their hybrids and composites. Emphasis is placed on strategic approaches which improve the value of the figure of merit for better thermoelectric performance at near room temperature via engineering material size, shape, composition, bandgap, etc. This review also describes the potential of thermoelectric materials for applications including self-powering wearable devices created by different approaches. Lastly, the underlying challenges and perspectives on the future development of thermoelectric materials are discussed.
Keywords: multinary alloys; near-room-temperature thermoelectric materials; silver-based chalcogenides; thermal energy harvesting; waste heat recovery.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
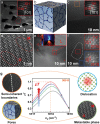
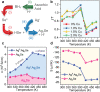
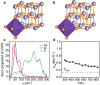

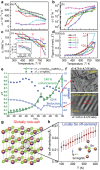
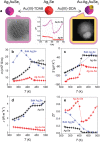
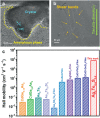
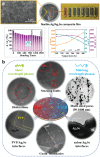
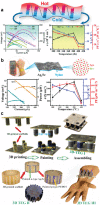
References
-
- Tritt T. M., Böttner H., Chen L., MRS Bull. 2011, 33, 366.
-
- Saini A., Kumar R., Kumar R., Introduction and Brief History of Thermoelectric Materials, Woodhead Publishing Series in Electronic and Optical Materials, Elsevier, Amsterdam: 2021.
-
- Vedernikov M. V., Iordanishvili E. K., in 17th Int. Conf. on Thermoelectrics , IEEE, Vol. 1, 1998, pp. 37–42.
-
- Goldsmid H. J., Douglas R. W., Br. J. Appl. Phys. 1954, 5, 386.
-
- Chasmar R. P., Stratton R., J. Electron. Sci. Technol. Control 1959, 7, 52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
