ICS/Ultra LABA in the Treatment of Obstructive Airway Diseases: A Consensus of Indian Experts
- PMID: 36285979
- PMCID: PMC9717339
- DOI: 10.3390/arm90050051
ICS/Ultra LABA in the Treatment of Obstructive Airway Diseases: A Consensus of Indian Experts
Abstract
Inhaled corticosteroid and ultra-long-acting beta-agonist (ICS/uLABA) combination is a recent advancement in the armamentarium against obstructive airways diseases (OADs). The combination of ICS/uLABA has several advantages, creating a favorable landscape for its utilization. Fluticasone furoate/vilanterol trifenatate (FF/Vi) is one such example of an ICS/uLABA. It offers several benefits from both drugs, such as a convenient once daily dosing schedule; high lipophilicity; high receptor affinity of fluticasone furoate along with high functional selectivity and a quick onset of action of vilanterol. However, the Global Initiative for Asthma (GINA) as well as the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines do not clearly define the positioning of ICS/uLABA compared to conventional ICS/LABAs. There are a few areas of uncertainty especially around the appropriate reliever strategy with ICS/uLABA in Asthma. The current consensus was planned with a group of Indian pulmonology experts to provide more clarity on the potential use of FF/Vi in Asthma and COPD. The clinical statements highlighted in this consensus manuscript address crucial clinical questions revolving around the efficacy and safety of FF/Vi as compared to conventional ICS/LABAs and identify the ideal patient profile for its use. This consensus paper also sheds light upon the appropriate reliever to be used along with FF/Vi in Asthma and the utilization of FF/Vi-based triple therapy in OADs. Expert recommendations mentioned in this paper will serve as guidance to pulmonologists as well as consultant physicians who are involved in providing care to OAD patients and will help them weigh the various factors that need to be taken into account while prescribing ICS/uLABA combination.
Keywords: Asthma; COPD; FF/Vi; ICS/ultra LABA; consensus; fluticasone furoate; vilanterol.
Conflict of interest statement
R. Dhar has nothing to disclose. D. Talwar has nothing to disclose. P. James has nothing to disclose. A Mishra has nothing to disclose. J. Vachaparambil has nothing to disclose. S. Patil is an employee of Glenmark Pharmaceuticals Ltd. N. Khatri is an employee of Glenmark Pharmaceuticals Ltd. S. Bhagat is an employee of Glenmark Pharmaceuticals Ltd. H. Barkate is an employee of Glenmark Pharmaceuticals Ltd.
Figures

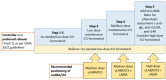
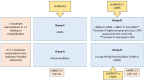
Similar articles
-
Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: An open-label, randomized, controlled trial.Respir Med. 2018 Aug;141:111-120. doi: 10.1016/j.rmed.2018.06.009. Epub 2018 Jun 9. Respir Med. 2018. PMID: 30053956 Clinical Trial.
-
Comparison of early effects of budesonide/formoterol maintenance and reliever therapy with fluticasone furoate/vilanterol for asthma patients requiring step-up from inhaled corticosteroid monotherapy.Pulm Pharmacol Ther. 2016 Apr;37:15-23. doi: 10.1016/j.pupt.2016.01.005. Epub 2016 Feb 2. Pulm Pharmacol Ther. 2016. PMID: 26850307 Clinical Trial.
-
Once-daily fluticasone furoate/vilanterol versus twice-daily fluticasone propionate/salmeterol in patients with asthma well controlled on ICS/LABA.J Asthma. 2018 Sep;55(9):984-993. doi: 10.1080/02770903.2017.1386214. Epub 2018 Apr 13. J Asthma. 2018. PMID: 28961020 Clinical Trial.
-
Fluticasone furoate and vilanterol inhalation powder for the treatment of chronic obstructive pulmonary disease.Expert Rev Respir Med. 2015 Feb;9(1):5-12. doi: 10.1586/17476348.2015.986468. Epub 2014 Dec 6. Expert Rev Respir Med. 2015. PMID: 25482512 Review.
-
Spotlight on fluticasone furoate/vilanterol trifenatate for the once-daily treatment of asthma: design, development and place in therapy.Drug Des Devel Ther. 2016 Dec 14;10:4047-4060. doi: 10.2147/DDDT.S113573. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 28008228 Free PMC article. Review.
Cited by
-
Socio-demographic environmental and clinical factors influencing asthma control in community pharmacies of Lahore Pakistan.Sci Rep. 2025 Mar 27;15(1):10587. doi: 10.1038/s41598-025-95373-4. Sci Rep. 2025. PMID: 40148570 Free PMC article.
References
-
- Slack R.J., Barrett V.J., Morrison V.S., Sturton R.G., Emmons A.J., Ford A.J., Knowles R.G. In Vitro Pharmacological Characterization of Vilanterol, a Novel Long-Acting β2-Adrenoceptor Agonist with 24-Hour Duration of Action. J. Pharm. Exp. 2013;344:218–230. doi: 10.1124/jpet.112.198481. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

