Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels
- PMID: 36286116
- PMCID: PMC9601853
- DOI: 10.3390/gels8100615
Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels
Abstract
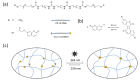
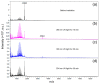
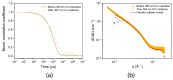
The design of photo-responsive supramolecular hydrogels based on coumarin dimerization and de-dimerization is described. The photo-responsive coumarin unit is chemically incorporated into an oligo(ethylene glycol) (OEG) bis-urea amphiphile that is capable of co-assembling with non-functionalized OEG amphiphile, to form supramolecular fibers. UV light with two different wavelengths (365 nm and 254 nm) is employed to induce a photo-reversible dimerization and de-dimerization process of coumarin units, respectively. The co-assembled solutions could be photo-crosslinked to induce a sol-to-gel transition through dimerization of coumarin with 365 nm UV light, and de-dimerization occurs with 254 nm UV light, to provide a weaker gel. In this system, the mechanical strength of supramolecular hydrogels can be tuned using the irradiation time, providing precise control of gelation in a supramolecular hydrogelator.
Keywords: bis-urea amphiphile; coumarin; photo-crosslinking; supramolecular hydrogels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


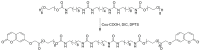




References
-
- Li Y., Zhu C., Dong Y., Liu D. Supramolecular Hydrogels: Mechanical Strengthening with Dynamics. Polymer. 2020;210:122993. doi: 10.1016/j.polymer.2020.122993. - DOI
-
- Li Y., Qin M., Cao Y., Wang W. Designing the Mechanical Properties of Peptide-Based Supramolecular Hydrogels for Biomedical Applications. Sci. China Phys. Mech. Astron. 2014;57:849–858. doi: 10.1007/s11433-014-5427-z. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

