Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation
- PMID: 36286140
- PMCID: PMC9601665
- DOI: 10.3390/gels8100639
Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation
Abstract
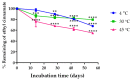
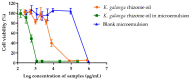
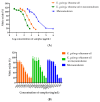
Long-term UV radiation exposure can induce skin disorders such as cancer and photoallergic reactions. Natural products have been considered as non-irritate and potential sunscreen resources due to their UV absorption and anti-inflammatory activities. This study aimed to evaluate the in vitro ultraviolet radiation protective effect and anti-inflammatory activity of K. galanga rhizome oil and microemulsions. The chemical components of K. galanga rhizome oil was analyzed via gas chromatography coupled with mass spectrometry. Microemulsions containing K. galanga rhizome oil were formulated using a phase-titration method. The microemulsion was characterized for droplet size, polydispersity index, and zeta potential, using a dynamic light-scattering technique. The physical and chemical stability of the microemulsion were evaluated via a dynamic light scattering technique and UV-Vis spectrophotometry, respectively. The UV protection of K. galanga rhizome oil and its microemulsion were investigated using an ultraviolet transmittance analyzer. The protective effect of K. galanga rhizome oil against LPS-induced inflammation was investigated via MTT and nitric oxide inhibitory assays. In addition, a hydrogel containing K. galanga rhizome oil microemulsion was developed, stored for 90 days at 4, 30, and 45 °C, and characterized for viscosity, rheology, and pH. The chemical degradation of the main active compound in the microemulsion was analyzed via UV-Vis spectrophotometry. The formulated O/W microemulsion contained a high loading efficiency (101.24 ± 2.08%) of K. galanga rhizome oil, suggesting a successful delivery system of the oil. The size, polydispersity index, and zeta potential values of the microemulsion were optimized and found to be stable when stored at 4, 30, and 45 °C. K. galanga rhizome oil and microemulsion demonstrated moderate sun protective activity and reduced the nitric oxide production induced by LPS in macrophage cells, indicating that microemulsion containing K. galanga rhizome oil may help protect human skin from UV damage and inflammation. A hydrogel containing K. galanga rhizome oil microemulsion was developed as a topical preparation. The hydrogel showed good physical stability after heating and cooling cycles and long-term storage (3 months) at 4 °C. The use of K. galanga rhizome oil as a natural sun-protective substance may provide a protective effect against inflammation on the skin. K. galanga rhizome oil microemulsion was successfully incorporated into the hydrogel and has the potential to be used as a topical sunscreen preparation.
Keywords: Zingiberaceae; essential oil; ethyl cinnamate; nitric oxide; sunscreen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources

