Hydrolytic Stability of Crosslinked, Highly Alkaline Diallyldimethylammonium Hydroxide Hydrogels
- PMID: 36286170
- PMCID: PMC9601492
- DOI: 10.3390/gels8100669
Hydrolytic Stability of Crosslinked, Highly Alkaline Diallyldimethylammonium Hydroxide Hydrogels
Abstract
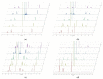
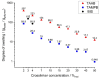
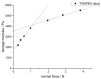
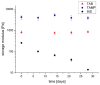
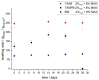
The aim of this study was to evaluate the persistence of alkaline hydrogels based on a common (N,N'-methylenebisacrylamide, BIS) and three recently published tetraallyl crosslinkers. Such hydrogels have been shown to be suitable materials for the rehabilitation of cementitious materials. Of the four crosslinkers under investigation, N,N,N',N'-tetraallylpiperazinium dibromide decomposed quickly in 1 m KOH solution and was not considered further. BIS showed the first signs of a decomposition after several days, while tetraallylammonium bromide and N,N,N',N'-tetraallyltrimethylene dipiperidine dibromide remained unaffected. In contrast to BIS, which suffers from low solubility in water, the two tetraallyl crosslinkers show unlimited miscibility with diallyldimethylammonium hydroxide solutions. For the study, gels with up to 50 wt % crosslinker were prepared. Of these, gels containing tetraallylammonium bromide always show the highest degrees of swelling, with a peak value of 397 g/g at a content of 2 wt %. Under accelerated ageing at 60 °C for 28 d, gels crosslinked with BIS ultimately turned liquid, while the storage modulus and the degree of swelling of the two tetraallyl-crosslinked gels remained unchanged. This indicates that alkaline gels can be suitable for long application periods, which are common for rehabilitation measures in the construction industry.
Keywords: copolymer; crosslinker; durability; hydrogel; hydrolysis; rheology; swelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
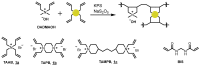
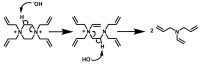

References
-
- Buchanan K.J., Hird B., Letcher T.M. Crosslinked poly(sodium acrylate) hydrogels. Polym. Bull. 1986;15:325–332. doi: 10.1007/BF00254851. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

