Descending neuron population dynamics during odor-evoked and spontaneous limb-dependent behaviors
- PMID: 36286408
- PMCID: PMC9605690
- DOI: 10.7554/eLife.81527
Descending neuron population dynamics during odor-evoked and spontaneous limb-dependent behaviors
Abstract
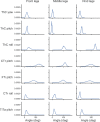
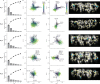
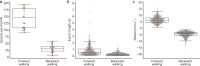
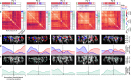
Deciphering how the brain regulates motor circuits to control complex behaviors is an important, long-standing challenge in neuroscience. In the fly, Drosophila melanogaster, this is coordinated by a population of ~ 1100 descending neurons (DNs). Activating only a few DNs is known to be sufficient to drive complex behaviors like walking and grooming. However, what additional role the larger population of DNs plays during natural behaviors remains largely unknown. For example, they may modulate core behavioral commands or comprise parallel pathways that are engaged depending on sensory context. We evaluated these possibilities by recording populations of nearly 100 DNs in individual tethered flies while they generated limb-dependent behaviors, including walking and grooming. We found that the largest fraction of recorded DNs encode walking while fewer are active during head grooming and resting. A large fraction of walk-encoding DNs encode turning and far fewer weakly encode speed. Although odor context does not determine which behavior-encoding DNs are recruited, a few DNs encode odors rather than behaviors. Lastly, we illustrate how one can identify individual neurons from DN population recordings by using their spatial, functional, and morphological properties. These results set the stage for a comprehensive, population-level understanding of how the brain's descending signals regulate complex motor actions.
Keywords: D. melanogaster; descending neuron; grooming; limb; neuroscience; population imaging; two-photon microscopy; walking.
© 2022, Aymanns et al.
Conflict of interest statement
FA, CC, PR No competing interests declared
Figures










References
-
- Aimon S, Cheng KY, Gjorgjieva J, Kadow ICG. Walking Elicits Global Brain Activity in Drosophila. bioRxiv. 2022 doi: 10.1101/2022.01.17.476660. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

