Enzyme Immobilization on Metal Organic Frameworks: the Effect of Buffer on the Stability of the Support
- PMID: 36286410
- PMCID: PMC9648341
- DOI: 10.1021/acs.langmuir.2c01630
Enzyme Immobilization on Metal Organic Frameworks: the Effect of Buffer on the Stability of the Support
Abstract
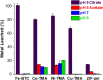
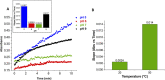
Metal organic frameworks (MOFs) have been used to encapsulate an array of enzymes in a rapid and facile manner; however, the stability of MOFs as supports for enzymes has not been examined in detail. This study examines the stability of MOFs with different compositions (Fe-BTC, Co-TMA, Ni-TMA, Cu-TMA, and ZIF-zni) in buffered solutions commonly used in enzyme immobilization and biocatalysis. Stability was assessed via quantification of the release of metals by inductively coupled plasma optical emission spectroscopy. The buffers used had varied effects on different MOF supports, with incubation of all MOFs in buffers resulting in the release of metal ions to varying extents. Fe-BTC was completely dissolved in citrate, a buffer that has a profound destabilizing effect on all MOFs analyzed, precluding its use with MOFs. MOFs were more stable in acetate, potassium phosphate, and Tris HCl buffers. The results obtained provide a guide for the selection of an appropriate buffer with a particular MOF as a support for the immobilization of an enzyme. In addition, these results identify the requirement to develop methods of improving the stability of MOFs in aqueous solutions. The use of polymer coatings was evaluated with polyacrylic acid (PAA) providing an improved level of stability. Lipase was immobilized in Fe-BTC with PAA coating, resulting in a stable biocatalyst with retention of activity in comparison to the free enzyme.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

