Physiologically Based Pharmacokinetic Modeling To Guide Management of Drug Interactions between Elexacaftor-Tezacaftor-Ivacaftor and Antibiotics for the Treatment of Nontuberculous Mycobacteria
- PMID: 36286508
- PMCID: PMC9664863
- DOI: 10.1128/aac.01104-22
Physiologically Based Pharmacokinetic Modeling To Guide Management of Drug Interactions between Elexacaftor-Tezacaftor-Ivacaftor and Antibiotics for the Treatment of Nontuberculous Mycobacteria
Abstract
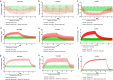
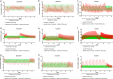
Nontuberculous mycobacteria (NTM) are the pathogens of concern in people with cystic fibrosis (pwCF) due to their association with deterioration of lung function. Treatment requires the use of a multidrug combination regimen, creating the potential for drug-drug interactions (DDIs) with cystic fibrosis transmembrane conductance regulator (CFTR)-modulating therapies, including elexacaftor, tezacaftor, and ivacaftor (ETI), which are eliminated mainly through cytochrome P450 (CYP) 3A-mediated metabolism. An assessment of the DDI risk for ETI coadministered with NTM treatments, including rifabutin, clofazimine, and clarithromycin, is needed to provide appropriate guidance on dosing. The CYP3A-mediated DDIs between ETI and the NTM therapies rifabutin, clarithromycin, and clofazimine were evaluated using physiologically based pharmacokinetic (PBPK) modeling by incorporating demographic and physiological "system" data with drug physicochemical and in vitro parameters. Models were verified and then applied to predict untested scenarios to guide continuation of ETI during antibiotic treatment, using ivacaftor as the most sensitive CYP3A4 substrate. The predicted area under the concentration-time curve (AUC) ratios of ivacaftor when coadministered with rifabutin, clofazimine, or clarithromycin were 0.31, 2.98, and 9.64, respectively, suggesting moderate and strong interactions. The simulation predicted adjusted dosing regimens of ETI administered concomitantly with NTM treatments, which required delayed resumption of the standard dose of ETI once the NTM treatments were completed. The dosing transitions were determined based on the characteristics of the perpetrator drugs, including the mechanism of CYP3A modulation and their elimination half-lives. This study suggests increased doses of elexacaftor/tezacaftor/ivacaftor 200/100/450 mg in the morning and 100/50/375 mg in the evening when ETI is coadministered with rifabutin and reduced doses of elexacaftor/tezacaftor 200/100 mg every 48 h (q48h) and ivacaftor 150 mg daily or a dose of elexacaftor/tezacaftor/ivacaftor 200/100/150 mg q72h when coadministered with clofazimine or clarithromycin, respectively. Importantly, the PBPK simulations provide evidence in support of the use of treatments for NTM in pwCF receiving concomitant dose-adjusted ETI therapy.
Keywords: PBPK modeling; cystic fibrosis; drug interactions; nontuberculosis mycobacteria.
Conflict of interest statement
The authors declare a conflict of interest. L.M.A. is an employee of Certara UK Limited (Simcyp Division). E.H., P.S.C., A.P.R., P.M.B. declared no competing interests for this work.
Figures
References
-
- Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS, US Cystic Fibrosis Foundation and European Cystic Fibrosis Society . 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–i22. 10.1136/thoraxjnl-2015-207360. - DOI - PMC - PubMed
-
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy KS, McCoy K, Donaldson S, Walker S, Chmiel J, Rubenstein R, Froh DK, Neuringer I, Jain M, Moffett K, Taylor-Cousar JL, Barnett B, Mueller G, Flume P, Livingston F, Mehdi N, Teneback C, Welter J, Jain R, Kissner D, Patel K, Calimano FJ, Johannes J, Daines C, Keens T, Scher H, Chittivelu S, Reddivalam S, Klingsberg RC, Johnson LG, Verhulst S, et al. 2019. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 394:1940–1948. 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
-
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. 10.1183/13993003.00535-2020. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

