Using Catalysis to Drive Chemistry Away from Equilibrium: Relating Kinetic Asymmetry, Power Strokes, and the Curtin-Hammett Principle in Brownian Ratchets
- PMID: 36286995
- PMCID: PMC9650702
- DOI: 10.1021/jacs.2c08723
Using Catalysis to Drive Chemistry Away from Equilibrium: Relating Kinetic Asymmetry, Power Strokes, and the Curtin-Hammett Principle in Brownian Ratchets
Abstract
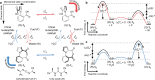
Chemically fueled autonomous molecular machines are catalysis-driven systems governed by Brownian information ratchet mechanisms. One fundamental principle behind their operation is kinetic asymmetry, which quantifies the directionality of molecular motors. However, it is difficult for synthetic chemists to apply this concept to molecular design because kinetic asymmetry is usually introduced in abstract mathematical terms involving experimentally inaccessible parameters. Furthermore, two seemingly contradictory mechanisms have been proposed for chemically driven autonomous molecular machines: Brownian ratchet and power stroke mechanisms. This Perspective addresses both these issues, providing accessible and experimentally useful design principles for catalysis-driven molecular machinery. We relate kinetic asymmetry to the Curtin-Hammett principle using a synthetic rotary motor and a kinesin walker as illustrative examples. Our approach describes these molecular motors in terms of the Brownian ratchet mechanism but pinpoints both chemical gating and power strokes as tunable design elements that can affect kinetic asymmetry. We explain why this approach to kinetic asymmetry is consistent with previous ones and outline conditions where power strokes can be useful design elements. Finally, we discuss the role of information, a concept used with different meanings in the literature. We hope that this Perspective will be accessible to a broad range of chemists, clarifying the parameters that can be usefully controlled in the design and synthesis of molecular machines and related systems. It may also aid a more comprehensive and interdisciplinary understanding of biomolecular machinery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Molecular Motors; Schliwa M., Ed.; Wiley-VCH, 2003.
-
- Howard J.Mechanics of Motor Proteins and the Cytoskeleton; Oxford University Press: Oxford, U.K., 2001.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

