Autophagy, cell death, and cytokines in K. pneumoniae infection: therapeutic perspectives
- PMID: 36287114
- PMCID: PMC9766508
- DOI: 10.1080/22221751.2022.2140607
Autophagy, cell death, and cytokines in K. pneumoniae infection: therapeutic perspectives
Abstract
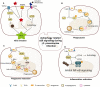
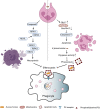
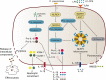
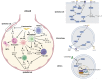
Klebsiella pneumoniae is a notorious nosocomial pathogen causing a wide range of infections. The increasing trend of antimicrobial resistance obtained by the species immensely highly challenges the clinical treatment, representing a large threat to the global health care network. In particular, the recent convergence of multidrug resistance and hypervirulence in K. pneumoniae further worsens clinical outcomes, resulting in high mortality. Developments of new therapeutics become urgent, and immunotherapy based on antagonizing the anti-immune strategies of pathogens is a promising strategy, which requires the understanding of immune evasion mechanism in the context of the host-pathogen interactions. However, the underlying mechanisms employed by K. pneumoniae to counteract host immune responses, especially autophagy and cell death, have not been systematically reviewed and discussed yet. This review aims to summarize the tremendous progress that has been made to illuminate the landscape of cell signalling triggered by K. pneumoniae infection, especially in aspects of manipulating autophagy, cell death, and cytokine production.
Keywords: Klebsiella pneumoniae; apoptosis; autophagy; cytokines; necrosis; pyroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Pyroptosis, apoptosis, and autophagy are involved in infection induced by two clinical Klebsiella pneumoniae isolates with different virulence.Front Cell Infect Microbiol. 2023 May 8;13:1165609. doi: 10.3389/fcimb.2023.1165609. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37223846 Free PMC article.
-
Klebsiella pneumoniae infection biology: living to counteract host defences.FEMS Microbiol Rev. 2019 Mar 1;43(2):123-144. doi: 10.1093/femsre/fuy043. FEMS Microbiol Rev. 2019. PMID: 30452654 Free PMC article. Review.
-
Antimicrobial polymers as therapeutics for treatment of multidrug-resistant Klebsiella pneumoniae lung infection.Acta Biomater. 2018 Sep 15;78:78-88. doi: 10.1016/j.actbio.2018.07.038. Epub 2018 Jul 20. Acta Biomater. 2018. PMID: 30031912
-
Klebsiella pneumoniae Reduces SUMOylation To Limit Host Defense Responses.mBio. 2020 Sep 29;11(5):e01733-20. doi: 10.1128/mBio.01733-20. mBio. 2020. PMID: 32994335 Free PMC article.
-
Hypervirulence and carbapenem resistance: two distinct evolutionary directions that led high-risk Klebsiella pneumoniae clones to epidemic success.Expert Rev Mol Diagn. 2019 Sep;19(9):825-837. doi: 10.1080/14737159.2019.1649145. Epub 2019 Aug 1. Expert Rev Mol Diagn. 2019. PMID: 31343934 Review.
Cited by
-
Proteome analysis, genetic characterization, and antibiotic resistance patterns of Klebsiella pneumoniae clinical isolates.AMB Express. 2024 May 9;14(1):54. doi: 10.1186/s13568-024-01710-7. AMB Express. 2024. PMID: 38722429 Free PMC article.
-
Artesunate Treats Aspergillus fumigatus Keratitis by Inhibiting Fungal Activity and Activating Autophagy Pathway to Reduce Corneal Inflammation.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):19. doi: 10.1167/iovs.66.11.19. Invest Ophthalmol Vis Sci. 2025. PMID: 40772662 Free PMC article.
-
Effect of SiHuangQingXinWan on Klebsiella pneumoniae-induced pneumonia: mechanistic insights.Front Pharmacol. 2024 Oct 15;15:1444439. doi: 10.3389/fphar.2024.1444439. eCollection 2024. Front Pharmacol. 2024. PMID: 39474608 Free PMC article.
-
Enhancing acute inflammatory and sepsis treatment: superiority of membrane receptor blockade.Front Immunol. 2024 Jul 16;15:1424768. doi: 10.3389/fimmu.2024.1424768. eCollection 2024. Front Immunol. 2024. PMID: 39081318 Free PMC article. Review.
-
Pyroptosis, apoptosis, and autophagy are involved in infection induced by two clinical Klebsiella pneumoniae isolates with different virulence.Front Cell Infect Microbiol. 2023 May 8;13:1165609. doi: 10.3389/fcimb.2023.1165609. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37223846 Free PMC article.
References
-
- Gu D, Dong N, Zheng Z, et al. . A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
