Asymmetric Total Synthesis of Havellockate
- PMID: 36287147
- PMCID: PMC9997676
- DOI: 10.1021/jacs.2c09583
Asymmetric Total Synthesis of Havellockate
Abstract
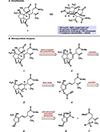
The first total synthesis of the furanobutenolide-derived cembranoid diterpenoid havellockate is disclosed. Our convergent strategy employs a Julia-Kocienski olefination to join two enantioenriched fragments to produce a diene that is subsequently used in a propiolic acid esterification/Diels-Alder cascade. This sequence generates the fused carbocyclic core of the natural product in short order. A challenging Zn-mediated Barbier allylation then forges the final C-C bond and also establishes two vicinal stereogenic centers. Finally, a Cu-catalyzed aerobic oxidation facilitates the formation of the β-hydroxybutanolide to complete the total synthesis.
Figures



References
-
- Carroll AR; Copp BR; Davis RA; Keyzers RA; Prinsep MR Marine Natural Products. Nat. Prod. Rep. 2022, 39, 1122–1171. - PubMed
-
- Li Y; Pattenden G Perspectives on the Structural and Biosynthetic Interrelationships Between Oxygenated Furanocembranoids and Their Polycyclic Congeners Found in Corals. Nat. Prod. Rep. 2011, 28, 1269–1310. - PubMed
-
- Tang B; Bray CD; Pattenden G A Biomimetic Total Synthesis of (+)-Intricarene. Tetrahedron Lett. 2006, 47, 6401–6404.
- Roethle PA; Hernandez PT; Trauner D Exploring Biosynthetic Relationships Among Furanocembranoids: Synthesis of (−)-Bipinnatin J, (+)-Intricarene, (+)-Rubifolide, and (+)-Isoepilophodione B. Org. Lett. 2006, 8, 5901–5904. - PubMed
- Stichnoth D; Kölle P; Kimbrough TJ; Riedle E; de Vivie-Riedle R; Trauner D Photochemical Formation of Intricarene. Nat. Commun. 2014, 5, 5597. For previous syntheses of the polycyclic furanobutenolide-derived norcembranoid scabrolide A, see: - PubMed
- Hafeman NJ; Loskot SA; Reimann CE; Pritchett BP; Virgil SC; Stoltz BM The Total Synthesis of (−)-Scabrolide A. J. Am. Chem. Soc. 2020, 142, 8585–8590 - PubMed
- Meng Z; Fürstner A Total Syntheses of Scabrolide A and Nominal Scabrolide B. J. Am. Chem. Soc. 2022, 144, 1528–1533. For the recent total synthesis of rameswaralide see: - PMC - PubMed
- Truax NJ; Ayinde S; Liu JO; Romo D Total Synthesis of Rameswaralide Utilizing a Pharmacophore-Directed Retrosynthetic Strategy. J. Am. Chem. Soc. 2022, in press. https://pubs.acs.org/doi/10.1021/jacs.2c08245. The total synthesis of ineleganolide was also recently disclosed: - DOI - PMC - PubMed
- Tuccinardi JP; Wood JL Total Synthesis of Complex Furanocembranoids. Presented at 47th National Organic Symposium, La Jolla, CA, June 26–June 30, 2022.
-
- Anjaneyulu ASR; Venugopal MJRV; Sarada P; Clardy J; Lobkovsky E Havellockate, A Novel Seco and Spiro Lactone Diterpenoid from the Indian Ocean Soft Coral Sinularia granosa. Tetrahedron Lett. 1998, 39, 139–142.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

