Macrophage depletion blocks congenital SARM1-dependent neuropathy
- PMID: 36287209
- PMCID: PMC9711884
- DOI: 10.1172/JCI159800
Macrophage depletion blocks congenital SARM1-dependent neuropathy
Abstract
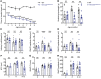
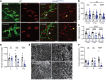
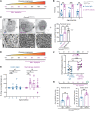
Axon loss contributes to many common neurodegenerative disorders. In healthy axons, the axon survival factor NMNAT2 inhibits SARM1, the central executioner of programmed axon degeneration. We identified 2 rare NMNAT2 missense variants in 2 brothers afflicted with a progressive neuropathy syndrome. The polymorphisms resulted in amino acid substitutions V98M and R232Q, which reduced NMNAT2 NAD+-synthetase activity. We generated a mouse model to mirror the human syndrome and found that Nmnat2V98M/R232Q compound-heterozygous CRISPR mice survived to adulthood but developed progressive motor dysfunction, peripheral axon loss, and macrophage infiltration. These disease phenotypes were all SARM1-dependent. Remarkably, macrophage depletion therapy blocked and reversed neuropathic phenotypes in Nmnat2V98M/R232Q mice, identifying a SARM1-dependent neuroimmune mechanism as a key driver of disease pathogenesis. These findings demonstrate that SARM1 induced inflammatory neuropathy and highlight the potential of immune therapy as a treatment for this rare syndrome and other neurodegenerative conditions associated with NMNAT2 loss and SARM1 activation.
Keywords: Macrophages; Mouse models; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

