Safety and efficacy of atezolizumab with rituximab and CHOP in previously untreated diffuse large B-cell lymphoma
- PMID: 36287231
- PMCID: PMC10125909
- DOI: 10.1182/bloodadvances.2022008344
Safety and efficacy of atezolizumab with rituximab and CHOP in previously untreated diffuse large B-cell lymphoma
Abstract
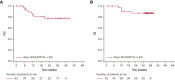
Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the current standard therapy for patients with diffuse large B-cell lymphoma (DLBCL) and is curative in ∼60% of patients. Atezolizumab is a humanized immunoglobulin G1 monoclonal antibody that targets programmed death-ligand 1 and has previously shown antitumor activity in several tumor types. In a phase 1b/2 trial (NCT02596971), we evaluated the safety and efficacy of atezolizumab in combination with R-CHOP (atezo-R-CHOP; for 6-8 cycles) in patients with previously untreated DLBCL. Patients achieving a complete response (CR) at the end of induction received consolidation therapy with atezolizumab on day 1 of each 21-day cycle for an additional 17 cycles. Overall, 42 patients with DLBCL were included in this analysis. The primary endpoint, CR rate at the end of induction, as assessed by an independent review committee (modified Lugano 2014 criteria), was 77.5% (95% confidence interval [CI], 64.0-87.7; n = 40). Investigator-assessed progression-free survival and overall survival at 3 years were 77.4% (95% CI, 59.7-88.0) and 87.2% (95% CI, 71.9-94.5), respectively. All treated patients experienced ≥1 adverse event (AE; 32 patients [76.2%] had grade 3-4 AE). One patient had a fatal AE (unconfirmed progressive multifocal leukoencephalopathy) that was considered related to atezolizumab and rituximab, and 17 patients (40.5%) experienced atezolizumab-related AEs of special interest. In previously untreated patients with DLBCL, atezo-R-CHOP demonstrated encouraging clinical efficacy and a safety profile consistent with the known toxicities of the individual drugs. This trial was registered at www.clinicaltrials.gov as #NCT02596971.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.Y. is employed by and has stock and other ownership interests in AstraZeneca; has received honoraria from Merck, F. Hoffmann-La Roche Ltd, Takeda, Janssen, AbbVie, Curis, and Epizyme; has a consulting or advisory role with BioPath Holdings Inc, Xynomic Pharma, Epizyme, F. Hoffmann-La Roche Ltd, Celgene, and HCM; has received research funding from Janssen, Curis, F. Hoffmann-La Roche Ltd, Genentech, Inc, Merck, Bristol Myers Squibb, Syndax; and other relationship with AstraZeneca. J.M.B. reports an advisory board role for Gilead, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, Bayer, AstraZeneca, AbbVie, Verastem, MorphoSys, Adaptive Biotechnologies, Epizyme, Kura, and Seattle Genetics, and has served on speaker's bureau for Seattle Genetics and Beigene. B.D.C. reports other fees from F. Hoffmann-La Roche Ltd, Genentech, Inc, and personal fees from Celgene, AbbVie, AstraZeneca, TG Therapeutics, Epizyme, Beigene, Karyopharm, MorphoSys, Symbios, Merck, Kite, GlaxoSmithKline, Janssen/Pharmacyclics, and Lilly, outside the submitted work. C.S.D. reports an advisory board/consultancy role for Celgene, F. Hoffmann-La Roche Ltd, Genentech, Inc, Bristol Myers Squibb, Merck, Seattle Genetics, Incyte, MorphoSys, and MEI Pharma. U.H.H. reports grants from F. Hoffmann-La Roche Ltd. E.A.H. reports research funding from F. Hoffmann-La Roche Ltd, Bristol Myers Squibb/Celgene, Merck KGaA, AstraZeneca; has served on advisory boards for F. Hoffmann-La Roche Ltd, Bristol Myers Squibb, Gilead, Novartis, AstraZeneca, Merck Sharp & Dohme, Janssen, Antigene, Specialised Therapeutics, and Beigene; and has served on the speaker's bureau for F. Hoffmann-La Roche Ltd, AstraZeneca, and Janssen. C.K. has served on speaker's bureau for Genentech, Inc, AstraZeneca, AbbVie, Beigene, Incyte, Kite, ADC Therapeutics, Karyopharm, and Seattle Genetics. I.S.L. has served on advisory boards for Seattle Genetics, Janssen Scientific, and Verastem, Inc. G.M. reports advisory board/consultancy fees from F. Hoffmann-La Roche Ltd, Incyte, and Janssen. U.V. has served on an advisory board for Janssen, Gilead, Celgene, Juno Therapeutics, Incyte, and Genmab, and received personal fees from F. Hoffmann-La Roche Ltd, Janssen, Gilead, and AbbVie. S.Y. has received travel assistance from Seattle Genetics. A.R. reports previous employment with Genentech, Inc and F. Hoffmann-La Roche Ltd. M.S. is employed by F. Hoffmann-La Roche Ltd. T.N. is employed by and holds equity in F. Hoffmann-La Roche Ltd. G.S. is employed by and owns nonvoting shares in F. Hoffmann-La Roche Ltd. J.P.S. reports a consultancy role with AbbVie, AstraZeneca, Beigene, Bristol Myers Squibb, Pfizer, Genentech, Inc. The remaining authors declare no competing financial interests.
The current affiliation for B.D.C. is Lymphoma Research Foundation, New York, NY.
The current affiliation for M.S. is Clinical Biomarkers, Translational Science Department, Arcus Biosciences, Hayward, CA.
Figures
References
-
- Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–616. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials