Microbial-induced Redox Imbalance in the Neonatal Lung Is Ameliorated by Live Biotherapeutics
- PMID: 36287630
- PMCID: PMC9989473
- DOI: 10.1165/rcmb.2021-0508OC
Microbial-induced Redox Imbalance in the Neonatal Lung Is Ameliorated by Live Biotherapeutics
Abstract
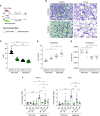
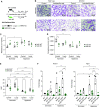
Bronchopulmonary dysplasia (BPD) is a common lung disease of premature infants. Hyperoxia exposure and microbial dysbiosis are contributors to BPD development. However, the mechanisms linking pulmonary microbial dysbiosis to worsening lung injury are unknown. Nrf2 (nuclear factor erythroid 2-related factor 2) is a transcription factor that regulates oxidative stress responses and modulates hyperoxia-induced lung injury. We hypothesized that airway dysbiosis would attenuate Nrf2-dependent antioxidant function, resulting in a more severe phenotype of BPD. Here, we show that preterm infants with a Gammaproteobacteria-predominant dysbiosis have increased endotoxin in tracheal aspirates, and mice monocolonized with the representative Gammaproteobacteria Escherichia coli show increased tissue damage compared with germ-free (GF) control mice. Furthermore, we show Nrf2-deficient mice have worse lung structure and function after exposure to hyperoxia when the airway microbiome is augmented with E. coli. To confirm the disease-initiating potential of airway dysbiosis, we developed a novel humanized mouse model by colonizing GF mice with tracheal aspirates from human infants with or without severe BPD, producing gnotobiotic mice with BPD-associated and non-BPD-associated lung microbiomes. After hyperoxia exposure, BPD-associated mice demonstrated a more severe BPD phenotype and increased expression of Nrf2-regulated genes, compared with GF and non-BPD-associated mice. Furthermore, augmenting Nrf2-mediated antioxidant activity by supporting colonization with Lactobacillus species improved dysbiotic-augmented lung injury. Our results demonstrate that a lack of protective pulmonary microbiome signature attenuates an Nrf2-mediated antioxidant response, which is augmented by a respiratory probiotic blend. We anticipate antioxidant pathways will be major targets of future microbiome-based therapeutics for respiratory disease.
Keywords: antioxidant; humanized microbiome; microbiota; neonatal lung injury; redox.
Figures




Comment in
-
Leveraging Microbial Symbiosis to Modulate Bronchopulmonary Dysplasia.Am J Respir Cell Mol Biol. 2023 Mar;68(3):235-236. doi: 10.1165/rcmb.2022-0415ED. Am J Respir Cell Mol Biol. 2023. PMID: 36346615 Free PMC article. No abstract available.
References
-
- Madurga A, Mizíková I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol . 2013;305:L893–L905. - PubMed
-
- Pammi M, Lal CV, Wagner BD, Mourani PM, Lohmann P, Luna RA, et al. Airway microbiome and development of bronchopulmonary dysplasia in preterm infants: a systematic review. J Pediatr . 2019;204:126–133.e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

