A novel RBD-protein/peptide vaccine elicits broadly neutralizing antibodies and protects mice and macaques against SARS-CoV-2
- PMID: 36287714
- PMCID: PMC9662039
- DOI: 10.1080/22221751.2022.2140608
A novel RBD-protein/peptide vaccine elicits broadly neutralizing antibodies and protects mice and macaques against SARS-CoV-2
Abstract
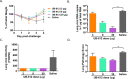
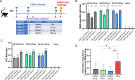
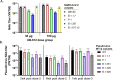
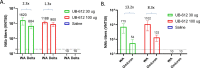
The development of safe and effective vaccines to respond to COVID-19 pandemic/endemic remains a priority. We developed a novel subunit protein-peptide COVID-19 vaccine candidate (UB-612) composed of: (i) receptor binding domain of SARS-CoV-2 spike protein fused to a modified single-chain human IgG1 Fc; (ii) five synthetic peptides incorporating conserved helper and cytotoxic T lymphocyte (Th/CTL) epitopes derived from SARS-CoV-2 structural proteins (three from S2 subunit, one from membrane and one from nucleocapsid), and one universal Th peptide; (iii) aluminum phosphate as adjuvant. The immunogenicity and protective immunity induced by UB-612 vaccine were evaluated in four animal models: Sprague-Dawley rats, AAV-hACE2 transduced BALB/c mice, rhesus and cynomolgus macaques. UB-612 vaccine induced high levels of neutralizing antibody and T-cell responses, in all animals. The immune sera from vaccinated animals neutralized the SARS-CoV-2 original wild-type strains and multiple variants of concern, including Delta and Omicron. The vaccination significantly reduced viral loads, lung pathology scores, and disease progression after intranasal and intratracheal challenge with SARS-CoV-2 in mice, rhesus and cynomolgus macaques. UB-612 has been tested in primary regimens in Phase 1 and Phase 2 clinical studies and is currently being evaluated in a global pivotal Phase 3 clinical study as a single dose heterologous booster.
Keywords: SARS-CoV-2; neutralizing antibody; non-human primate; protection; subunit vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


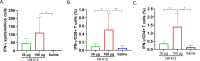

References
-
- Control ECfDPa. Epidemiological update: SARS-CoV-2 Omicron sub-lineages BA.4 and BA.5. . https://wwwecdceuropaeu/en/news-events/epidemiological-update-sars-cov-2.... 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
