Ion-Molecule Rate Constants for Reactions of Sulfuric Acid with Acetate and Nitrate Ions
- PMID: 36287779
- PMCID: PMC9661533
- DOI: 10.1021/acs.jpca.2c02072
Ion-Molecule Rate Constants for Reactions of Sulfuric Acid with Acetate and Nitrate Ions
Abstract
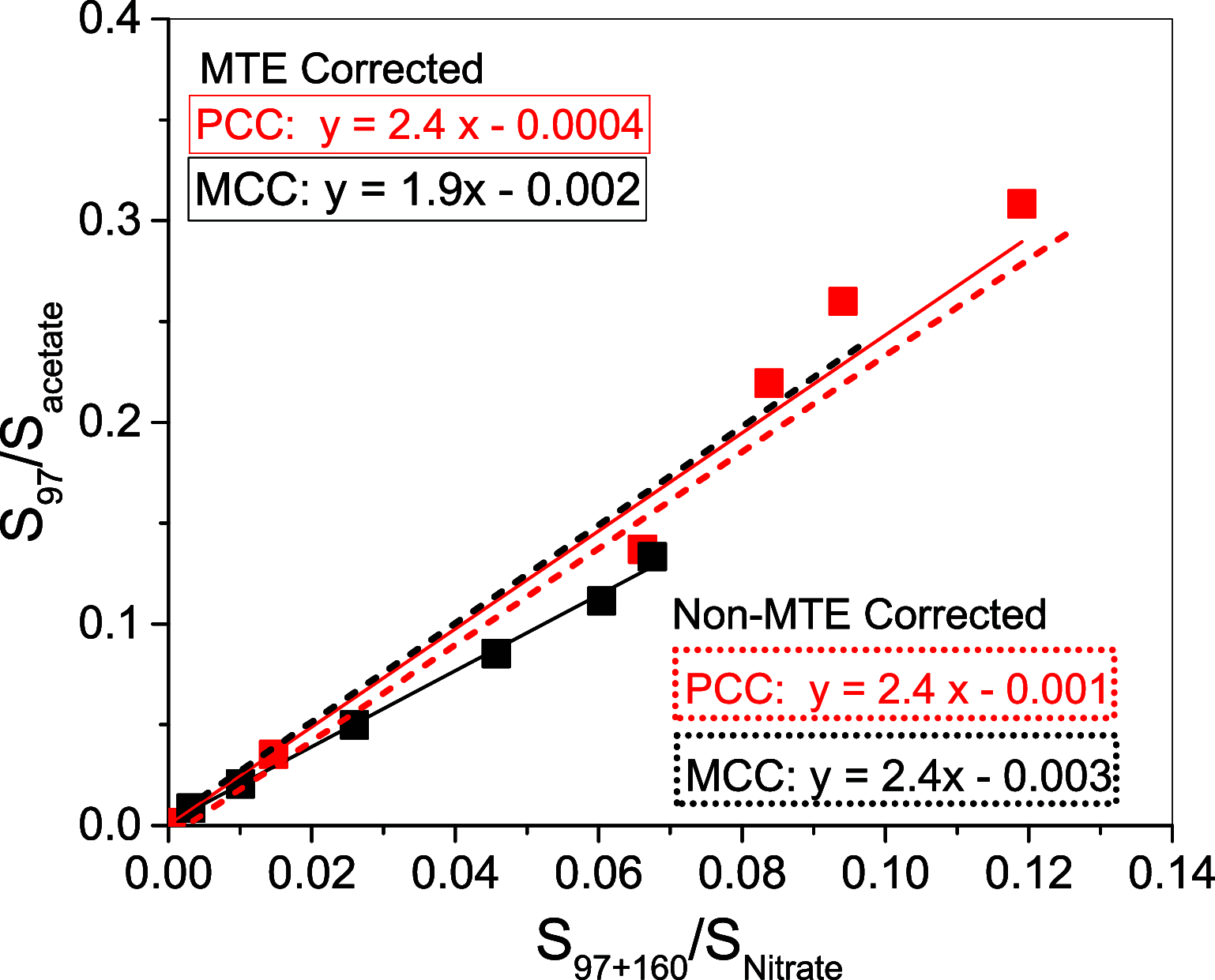
Atmospheric nucleation from precursor gases is a significant source of cloud condensation nuclei in the troposphere and thus can affect the Earth's radiative balance. Sulfuric acid, ammonia, and amines have been identified as key nucleation precursors in the atmosphere. Studies have also shown that atmospheric ions can react with sulfuric acid to form stable clusters in a process referred to as ion-induced nucleation (IIN). IIN follows similar reaction pathways as chemical ionization, which is used to detect and measure nucleation precursors via atmospheric pressure chemical ionization mass spectrometers. The rate at which ions form clusters depends on the ion-molecule rate constant. However, the rate constant varies based on the ion composition, which is often not known in the atmosphere. Previous studies have examined ion-molecule rate constants for sulfuric acid and nitrate ions but not for other atmospherically relevant ions like acetate. We report the relative rate constants of ion-molecule reactions between nitrate and acetate ions reacting with sulfuric acid. The ion-molecule rate constant for acetate and sulfuric acid is estimated to be a factor of 1.9-2.4 times higher than that of the known rate constant for nitrate and sulfuric acid. Using quantum chemistry, we find that acetate has a higher dipole moment and polarizability than nitrate. This may contribute to an increase in the collision cross-sectional area between acetate and sulfuric acid and lead to a greater reaction rate constant than nitrate. The ion-molecule rate constant for acetate with sulfuric acid will help quantify the contribution of acetate ions to atmospheric ion-induced new particle formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Merikanto J.; Spracklen D. V.; Mann G. W.; Pickering S. J.; Carslaw K. S. Impact of Nucleation on Global CCN. Atmospheric Chemistry and Physics 2009, 9 (21), 8601–8616. 10.5194/acp-9-8601-2009. - DOI
-
- Gordon H.; Kirkby J.; Baltensperger U.; Bianchi F.; Breitenlechner M.; Curtius J.; Dias A.; Dommen J.; Donahue N. M.; Dunne E. M.; et al. Causes and Importance of New Particle Formation in the Present-Day and Preindustrial Atmospheres. Journal of Geophysical Research: Atmospheres 2017, 122 (16), 8739–8760. 10.1002/2017JD026844. - DOI
-
- Spracklen D. V.; Carslaw K. S.; Kulmala M.; Kerminen V.-M.; Sihto S.-L.; Riipinen I.; Merikanto J.; Mann G. W.; Chipperfield M.; et al. P.; Wiedensohler, A.; et al. Contribution of Particle Formation to Global Cloud Condensation Nuclei Concentrations. Geophys. Res. Lett. 2008, 35 (6), L06808. 10.1029/2007GL033038. - DOI
-
- Wang M.; Penner J. E. Aerosol Indirect Forcing in a Global Model with Particle Nucleation. Atmospheric Chemistry and Physics 2009, 9 (1), 239–260. 10.5194/acp-9-239-2009. - DOI
-
- Yu F.; Luo G. Simulation of Particle Size Distribution with a Global Aerosol Model: Contribution of Nucleation to Aerosol and CCN Number Concentrations. Atmospheric Chemistry and Physics 2009, 9 (20), 7691–7710. 10.5194/acp-9-7691-2009. - DOI
LinkOut - more resources
Full Text Sources

