3D Single-Breath Chemical Shift Imaging Hyperpolarized Xe-129 MRI of Healthy, CF, IPF, and COPD Subjects
- PMID: 36287814
- PMCID: PMC9607398
- DOI: 10.3390/tomography8050215
3D Single-Breath Chemical Shift Imaging Hyperpolarized Xe-129 MRI of Healthy, CF, IPF, and COPD Subjects
Abstract
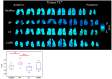
3D Single-breath Chemical Shift Imaging (3D-SBCSI) is a hybrid MR-spectroscopic imaging modality that uses hyperpolarized xenon-129 gas (Xe-129) to differentiate lung diseases by probing functional characteristics. This study tests the efficacy of 3D-SBCSI in differentiating physiology among pulmonary diseases. A total of 45 subjects-16 healthy, 11 idiopathic pulmonary fibrosis (IPF), 13 cystic fibrosis (CF), and 5 chronic obstructive pulmonary disease (COPD)-were given 1/3 forced vital capacity (FVC) of hyperpolarized Xe-129, inhaled for a ~7 s MRI acquisition. Proton, Xe-129 ventilation, and 3D-SBCSI images were acquired with separate breath-holds using a radiofrequency chest coil tuned to Xe-129. The Xe-129 spectrum was analyzed in each lung voxel for ratios of spectroscopic peaks, chemical shifts, and T2* relaxation. CF and COPD subjects had significantly more ventilation defects than IPF and healthy subjects, which correlated with FEV1 predicted (R = -0.74). FEV1 predicted correlated well with RBC/Gas ratio (R = 0.67). COPD and IPF had significantly higher Tissue/RBC ratios than other subjects, longer RBC T2* relaxation times, and greater RBC chemical shifts. CF subjects had more ventilation defects than healthy subjects, elevated Tissue/RBC ratio, shorter Tissue T2* relaxation, and greater RBC chemical shift. 3D-SBCSI may be helpful in the detection and characterization of pulmonary disease, following treatment efficacy, and predicting disease outcomes.
Keywords: COPD; MRI; cystic fibrosis; hyperpolarized xenon-129; idiopathic pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

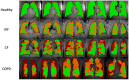






References
-
- Di Nardo F., Laurenti P. Respiratory Diseases and Health Disorders Related to Indoor and Outdoor Air Pollution. In: Boccia S., Villari P., Ricciardi W., editors. A Systematic Review of Key Issues in Public Health. Springer International Publishing; Berlin/Heidelberg, Germany: 2015. pp. 109–127. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

